
(1)
THE CHARACTERIZATION AND TESTING OF CANDIDATE
IMMOBILIZATION FORMS FOR THE
DISPOSAL OF PLUTONIUM
D. M. Strachan, A. J. Bakel, E. C. Buck, D. B. Chamberlain, J. A.
Fortner,
C. J. Mertz, S.F. Wolf
Argonne National Laboratory
W. L. Bourcier, B. B. Ebbinghaus, H. F. Shaw, R. A. Van Konynenburg
Lawrence Livermore National Laboratory
B. P. McGrail, J. D. Vienna
Pacific Northwest National Laboratory
J. C. Marra, D. K. Peeler
Savannah River Technology Center
ABSTRACT
Two candidate immobilization forms for the disposal of surplus weapons-usable Pu, glass and ceramic, were tested and characterized to provide sufficient data for an informed selection of one form for further R&D. Laboratory testing of each form was performed to provide data on critical issues such as Pu and neutron absorber solubility in the immobilization form, thermal stability, potential separation of absorber and Pu, and the long-term behavior of the materials in contact with water. Testing methods included MCC-1 tests, product consistency tests (methods A and B), unsaturated "drip" tests, vapor hydration tests, single-pass flow-through tests, and pressurized unsaturated flow tests. Both candidate immobilization forms were found to have very low dissolution rates but the ceramic reacted more slowly than the glass in both static and flow tests, at least at early times. This finding, in part, led to the selection of the ceramic as the preferred material for the immobilization of excess weapons-usable plutonium.
INTRODUCTION
Candidate immobilization forms for the disposal of surplus weapons-usable Pu are being tested and characterized. The goal of the testing program was to provide sufficient data that, by August 1997, an informed selection of a single immobilization form could be made. Two forms have been under consideration for the past two years: glass and ceramic. In August 1997, the Department of Energy (DOE) selected ceramic for plutonium disposition, halting further work on the glass material. Development and production R&D will now focus on the ceramic immobilization form.
The two candidate immobilization forms, glass and ceramic, have been under development to accommodate the anticipated feed streams of surplus plutonium. A lanthanide-borosilicate glass (LaBS) has been developed at the Savannah River Technology Center. This glass is an alkali-free, rare-earth rich borosilicate glass into which 10 mass% or more actinides can be dissolved.1 The composition of the reference LaBS glass is reported in Table 1. The LaBS glass, formulated to dissolve about 10 mass % Pu, has a density of 3600 kg/m3. In the LaBS glass, boron, gadolinium, and hafnium are neutron absorbers to control nuclear criticality. The glass is formulated so that an infinite array is criticality safe. The ceramic is under development at Lawrence Livermore National Laboratory and is based on the Synroc concept.2 It is a titanate-based ceramic, mainly composed of zirconolite with secondary phases of pyrochlore, Ba-hollandite, and rutile. The zirconolite and pyrochlore phases are the hosts for the Pu and the neutron absorbers Gd and Hf. The ceramic phase assemblage is given in Table 1. The ceramic, formulated to dissolve about 12 mass % Pu, has a density of 5500 kg/m3. As with the glass, the ceramic is formulated to be criticality safe when stacked in an infinite array.
Table 1. Compositions of LaBS and Ceramic Formulations
LaBS Formulation* |
Ceramic Formulation |
||
Component |
wt% |
||
Al2O3 |
17.9 |
Major Phases |
Zirconolite [CaZrTi2O7] |
B2O3 |
9.7 |
Pyrochlore [Gd2Ti2O7] |
|
Gd2O3 |
10.8 |
||
HfO2 |
5.5 |
Minor Phases |
Rutile (TiO2) |
PuO2 |
10.0 |
Hollandite ([Ba(Al2Ti6O16)] |
|
La2O3 |
6.8 |
Perovskite [CaTiO3] |
|
Nd2O3 |
6.9 |
Ca-Al-titanates |
|
SiO2 |
24.3 |
||
SrO2 |
2.1 |
||
UO3 |
3.0 |
||
Impurities, as oxides |
3.0 |
||
* Glass composition based upon the 50 metric ton plutonium disposition option.
The same analytical techniques are used to characterize unaltered samples and samples altered in dissolution tests. These techniques include optical, scanning electron, and transmission electron microscopies. At Argonne National Laboratory, small-particle handling techniques and the ultramicrotome are used to prepare samples for the TEM. For both candidate immobilization forms, the analyses are used to characterize the material for the presence of crystalline phases and amorphous material. Crystalline materials, either in the untested immobilization form or in the alteration products from testing, are characterized with respect to morphology, crystal structure, and composition. The goal of these analyses is to provide data on critical issues such as Pu and neutron absorber solubility in the immobilization form, thermal stability, potential separation of absorber and Pu, and the long-term behavior of the materials.
A complete set of dissolution and alteration tests has been performed on the candidate materials. The tests include MCC-1 tests,3 product consistency tests (methods A and B),4 unsaturated "drip" tests5 , vapor hydration tests,6 single-pass flow-through tests,7,8 and pressurized unsaturated flow tests.9
In the MCC-1 test,3 a monolith is placed in demineralized water at 90°C for a short period, typically three days. The surface area-to-volume ratio (S/V) in this test is 10 m-1. The purpose of this test is to provide an estimate of the forward rate of waste form reaction. This information bounds the rate of the waste-form reaction under a specific test condition but does not provide useful information on the release of Pu or the neutron absorber Gd, because of the low solution concentrations for these elements.
The product consistency test, method A (PCT-A),4 is a 7 day test with demineralized water at 90°C and with a powdered specimen at a S/V of 2000 m-1 in a stainless steel test vessel. This test is used for the high-level waste (HLW) glass currently being produced at the Defense Waste Processing Facility (DWPF) as a measure for product consistency. Product consistency is demonstrated by showing that the durability of the DWPF glasses being produced are better than a benchmark form as measured by the PCT.
In the PCT-B,4 a powder (-100+200 mesh) is placed in demineralized water at 90°C for variable time periods in a stainless steel test vessel. The S/V for this test is 20 000 m-1. This test is used to evaluate of the waste form reaction at high S/V and static conditions that increase the concentration of glass components in the leachate and, therefore, accelerate the onset of alteration phase formation. This information provides an indication of the long-term Pu and Gd release to solution and also can be used to infer the mechanism(s) of waste-form reaction.
The vapor hydration test (VHT)6 is a test of variable duration in which a monolithic specimen is used. The test temperatures that have been used range from 70°C to 260°C. Water is added to the test vessel such that at the test temperature no liquid water remains. This humid environment ensures that little water accumulates on the surface of the specimen, hence, high S/V. The test provides qualitative information on the tendency for alteration phases to form on materials. This test allows us to evaluate the distribution of Pu and Gd within the alteration phases, if applicable.
The single pass flow test7,8 is a test in which a solution is passed over/through a powdered or monolithic specimen. The solution may contain buffers to control the pH. The vessel with the specimen is placed in an oven or bath at controlled temperature. Typically, the temperature is between 25° C and 90°C. The response of the specimen to a flowing solution is measured. To measure the forward dissolution rate of a material, the flow rate must be high enough that the dissolution rate is independent of the flow rate. Two test designs were used and are described elsewhere.7,8
The pressurized unsaturated flow (PUF) test9 is an interaction test that employs granulated materials in a column arrangement. Any aqueous solutionmay be pumped through the sample, but in the studies reported here, demineralized water was used. The purpose of this test is to measure the dissolution rate of either the glass or the ceramic alone or with other potential waste package components under unsaturated flow. Materials may be combined in the column to study materials interactions. Hydraulic properties are also evaluated in this test.
In the last type of test, the drip test,5 a solution, typically J-13 well water that has been equilibrated with tuff,10 is dripped on a monolith of the test material. Temperatures are typically 90°C, and drip rates are about 25 m L/d. The purpose of these tests is to measure dissolution under slow-flow, unsaturated conditions.
Normalized elemental mass losses were calculated on the bases of the analyzed or target glass and ceramic compositions, the geometric surface area, and the solution composition after testing. The equation used to calculate normalized elemental mass loss is
 |
(1) |
where NLi is the normalized elemental mass loss in g/m2, Ci is the concentration of element i in the leachant and the acid strip of the test vessel in g/m3, Ci° is the concentration of element i in the initial leachant solution in g/m3, S is the surface area of the glass in m2, V is the volume of the solution in m3, and fi is the weight fraction of element i in the glass. The MCC-1 and PCT normalized mass losses include both the solution concentrations and the results of an acid strip of the test vessels. Because the length of the static tests was short, the dissolution rate was calculated by dividing the NLi by the test duration. In flow tests, however, the dissolution rate was calculated from the flow rate, the concentration of material in solution, and the time between samplings.
RESULTS AND DISCUSSION
To consolidate the results from dissolution testing, we plot the results from all the static tests as the normalized elemental mass loss (NLi) against the product of the surface-to-volume ratio (S/V) and time (t).
Results for the glass and ceramic are summarized in Figures 1 and 2, respectively. For the ceramic, there is no element that can be used to monitor the behavior of the bulk of the ceramic. This is because all elements from the ceramic in the leachate are suspect of having their concentrations controlled by the solubility of alteration products and/or the compounds that comprise the ceramic. As the material dissolves, the pH increases from 5.5 of demineralized water to 8.7 (Figure 1). As the pH increases, the NLi for all elements that were measurable decreased. These results usually indicate the precipitation of mineral phases. However, only calcite (CaCO3) and calcium sulfate (CaSO4) were found during examination of the test specimens in the microscope. Since the acid strip fraction is included in the calculation of the NLi values, the different values for NLi suggest incongruent dissolution of the ceramic.
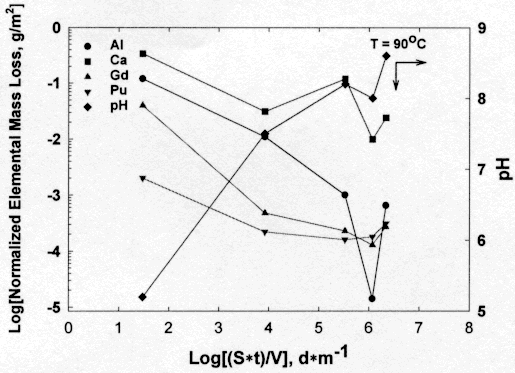
Figure 1. Normalized elemental mass loss for the Pu-ceramic at 90oC in demineralized water. The results are from MCC-1 and PCT-A, B.
Although similar trends were observed for the LaBS glass (Figure 2) constituents, it is unlikely that insoluble phases that contain boron form in these tests. Hence, the MCC-1, data at 3 days are suspect, and it is unlikely that the NLB values decrease with time for MCC-1. The PCT results indicate that the NL values for all elements are nearly invariant with time. Since all of the other NLi values are lower than NLB, insoluble minerals must be forming for these elements. In comparison, the ceramic releases about 100 times less material into solution than does the glass, even though the solution pH is nearly the same for ceramic and glass in MCC-1 and PCT.
In single-pass flow tests, results from Lawrence Livermore National Laboratory (LLNL) and Pacific Northwest National Laboratory (PNNL) can be compared. In Figure 3, results from PNNL for LaBS glass are shown for the elements Al and Si. Two forms of the glass were tested, one containing Ce and one Pu. The results for the forward rates are similar to other silicate-based glasses both in magnitude and pH response. Test results for the glass (PNNL) and the ceramic (LLNL) at a test temperature of 70°C are shown in Figure 4. The results for the LaBS glass are roughly 100 times higher than for the ceramic. Because the solubility of the constituent elements in the ceramic are very low, only a limited number of data are above the analytical detection limits.
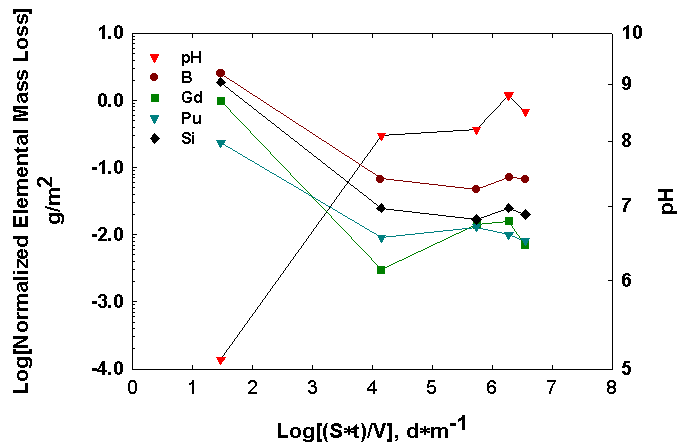
Figure 2. Normalized elemental mass loss for the LaBS glass at 90oC. The results are from MCC-1 and PCT-A, B.
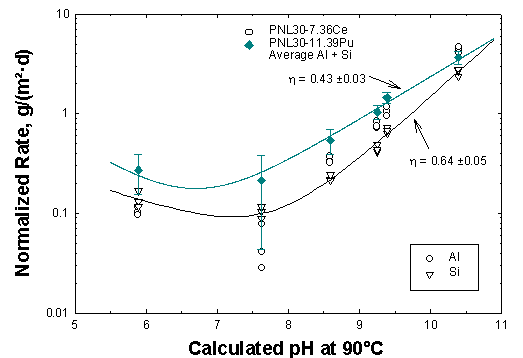
Figure 3. Dissolution rates from single pass flow tests with LaBS glasses.
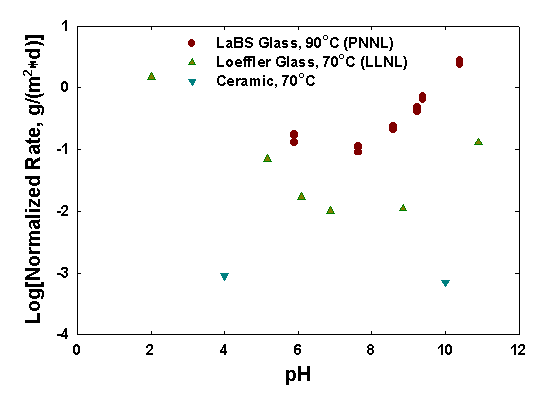
Figure 4. Dissolution rates from single pass flow tests with the Pu-ceramic and LaBS glass. (The Loeffler glass is an earlier version of the LaBS glass.)
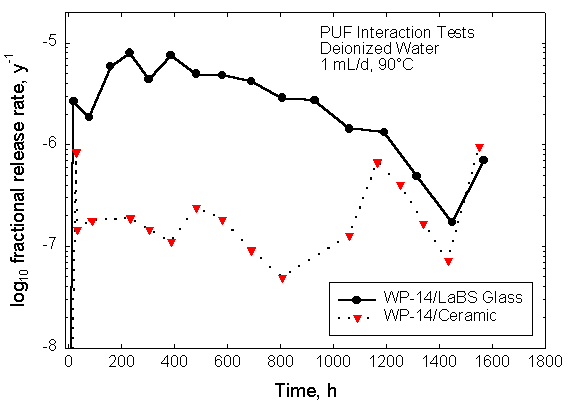
Figure 5. Results from the pressurized unsaturated flow experiments with the Pu-ceramic in demineralized water at 90oC.
Results from the PUF tests are shown in Figure 5 for a Ce-doped ceramic. The important conclusions are summarized here. As the reactions in the column take place, solution properties change. After some initial period, the pH, conductivity, and column saturation reach steady state. As the glass or ceramic reacts with solution, the solution concentrations increase. At some point, mineral phases precipitate. This event is evident from the changes in solution concentrations, pH, conductivity, and column saturation. The PUF data indicate also that the ceramic dissolves about 10 times slower than does the glass.
Use of the vapor hydration test yields the mineral phases that may form at long times in static tests. Mazer and co-workers11 have shown that applying this test to natural glasses and tektites will yield the same alteration minerals as found in nature. After this test was completed with ceramic and glass, the surfaces of the materials were examined. The ceramic material does not react very rapidly. Consequently, the reaction layers are very thin and not evenly distributed over the surface. These layers are too thin to be detected in the scanning electron microscope, for which sample preparation is much less aggressive. Anatase, iron hydroxides/hydrated oxides, and silica are found on the surface of the ceramic test samples (Figure 6). On the LaBS glass, a thicker la
yer is observed that consists mainly of clay-like material (Figure 7). A plutonium-rare earth silicate, possibly (Pu,RE)SiO4 [RE = all rare earths in the glass], was observed on the surface of and within the gel layer on the glass (Figure 7).
|
|
|
Figure 7. Results from vapor phase hydration of LaBS glass at 200oC for 56 days (a = Pu,RE silicate; b = gel layer; c = glass). |
CONCLUSIONS
A comprehensive suite of initial dissolution tests has been applied to the two candidate immobilization forms, a zirconolite-based ceramic and a lanthanum-borosilicate glass (LaBS). Both materials dissolve at a very low rate, with the ceramic consistently dissolving more slowly than the glass in both static and flow tests. This findings, in part, led to the selection of the ceramic as the material for the immobilization of excess weapons-usable plutonium. The results also indicate that the Gd and Pu have different dissolution behaviors and may become separated with time as the immobilization form dissolves and the Gd and Pu are transported.
Over the next several years, many tests will be performed on the ceramic material. The composition of the ceramic will be changed to include more uranium, thus forcing the predominant crystalline phase to be pyrochlore. This change is being made, in part, because the source of the plutonium to be immobilized will contain about as much uranium as plutonium. An added benefit will be the change from a monoclinic crystal type (zirconolite) to cubic (pyrochlore). The benefit will be realized in the potential for decreased microcracking during radiation damage, which will, over time, cause the material to become metamict (amorphous). Microcracking would cause the substantial increases to the accessible surface area with a concomitant increase in the fractional release of material to the groundwater.
Although single-pass flow tests have been performed with the pyrochlore material, these results are not reported here. However, the pyrochlore material is not expected to have a dissolution rate significantly different from the zirconolite. Initial results seem to support this assumption. Some of the alteration minerals that result from the dissolution of the ceramic may change. Of particular interest is the fate of the neutron absorbers. If, as has been seen for Zr, Hf becomes associated with the Pu, then the criticality concerns are lessened. One of the main concerns for the ceramic material is the presence of an amorphous or glassy phase at the grain boundaries. These phases tend to have a lower durability than the crystalline phases that make up the ceramic.
REFERENCES