DEVELOPMENT OF THE PLUTONIUM OXIDE
VITRIFICATION SYSTEM (U)
K. M. Marshall, J. C. Marra, J. T. Coughlin, T. B. Calloway
R. F. Schumacher, J. R. Zamecnik, and J. M. Pareizs
Savannah River Technology Center
ABSTRACT
Repository disposal of plutonium in a suitable, immobilized form is being considered as one option for the disposition of surplus weapons-usable plutonium. Accelerated development efforts were completed in 1997 on two potential immobilization forms to facilitate downselection to one form for continued development. The two forms studied were a crystalline ceramic based on Synroc [1] technology and a lanthanide borosilicate (LaBS) glass [2].
As part of the glass development program, melter design activities and component testing were completed to demonstrate the feasibility of using glass as an immobilization medium. A prototypical melter was designed and built in 1997. The melter vessel and drain tube were constructed of a Pt/Rh alloy. Separate induction systems were used to heat the vessel and drain tube. A Pt/Rh stirrer was incorporated into the design to facilitate homogenization of the melt. Integrated powder feeding and off-gas systems completed the overall design. Concurrent with the design efforts, testing was conducted using a plutonium surrogate LaBS composition in an existing (near-scale) melter to demonstrate the feasibility of processing the LaBS glass on a production scale. Additionally, the drain tube configuration was successfully tested using a plutonium surrogate LaBS glass.
INTRODUCTION
In the aftermath of the Cold War, the United States has taken the lead in technology development for fissile materials disposition to promote global non-proliferation. In support of this mission, the U. S. Department of Energy’s (DOE) Office of Fissile Materials Disposition (OFMD) has been charged with providing technical support for evaluation of options for the disposition of the excess fissile materials manufactured under the nation’s defense programs.
One option for plutonium disposition is immobilization using the can-in-canister concept. In this process, plutonium will be immobilized in a durable form and placed in small stainless steel cans.
Several of these cans will then be placed in a rack which will be positioned in a large stainless steel canister. The large canister will then be filled with high-level radioactive waste glass which will surround the small plutonium bearing cans and act as a high radiation, non-proliferation barrier.
Vitrification is a proven immobilization technology and is currently being used for the immobilization of high-level radioactive waste at the Savannah River Site. For the past two years, the site’s Defense Waste Processing Facility (DWPF) has been successfully vitrifying high-level radioactive wastes associated with weapons processing at SRS. A natural extrapolation of this technology is the immobilization of other radioactive materials, such as plutonium.
A lanthanide borosilicate (LaBS) glass was developed as a candidate plutonium immobilization form. The development efforts lead to a composition capable of incorporating in excess of 10 wt % plutonium into a homogeneous LaBS glass [2]. The durability of this glass was shown to be almost two orders of magnitude better than typical high-level waste glass compositions as measured by the Product Consistency Test (PCT) [3].
Platinum lined, bottom pour glass melters are attractive candidates for processing the LaBS glass for several reasons. High processing temperatures (~1500° C) are required to achieve the necessary plutonium loading in the glass, and at these processing temperatures platinum is a viable glass contact material. Additionally, platinum is not readily wetted by molten glasses so material hold-up in the melter upon draining is minimized. This is an important attribute from criticality and material accountability perspectives. Platinum melters are also routinely used in the commercial glass industry, and there is a great deal of process knowledge and data available on their usage.
Platinum melters can be heated in essentially three ways: indirect heating, direct resistance heating, and induction heating. All three of these methods adequately provide the necessary heat for glass melting and there is essentially little to differentiate between the three methods. Induction heated melters were selected and were developed for this application primarily because of the availability of commercial units of the size necessary for the production process. This helped minimize development efforts and provided additional process knowledge and confidence.
To support an accelerated plutonium immobilization form downselection process, vitrification system development efforts were completed in 1997. A melter system was designed and built as a prototype for an integrated production system. Concurrent with these efforts, melter system component testing with plutonium surrogate LaBS glass was completed to demonstrate the processability of the LaBS composition.
RESULTS AND DISCUSSION - MELTER DESIGN
The final plutonium melter system design centers on a cylindrical platinum vessel (Figures 1 and 2). A drain tube is welded to the bottom of the vessel, and the flow of glass from the tube is started and stopped by means of air jets aimed at the molten stream. A stirrer is incorporated into the melter to assist in homogenizing the glass. The stirrer can be easily lowered and raised from the melt pool, and is designed to withstand the high-temperature environment and expected glass viscosities.
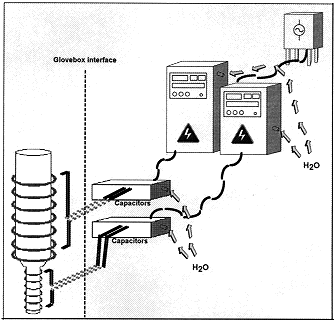
The vessel is heated inductively by a helical, water-cooled copper coil surrounding its length (Figure 1). The drain tube is heated inductively, as well, by a similar coil. The induction coils are powered by separate radio frequency (RF) power supplies that are components of a turnkey system. The RF range on the power supplies is from 50 to 200 kHZ. The power supplies self-tune to the optimal frequencies for heating the vessel and drain tube. They have the ability to be installed remote from the vessel, up to 200 feet away.

Fig. 1. Schematic of the melter system showing the induction heaters.
Insulation for the melter is in the form of a refractory textile sock which is slipped over the vessel. The wrapped vessel is then slipped into a rigid sleeve of insulation. The rigid cylindrical sleeve fits snugly against the induction coils. The resulting protected unit is then compact and easy to repair, with the sock allowing for expansion, and the rigid sleeve protecting against radial creep.
A plenum is designed to fit over and connect the top of the melter to the offgas line. It contains a number of sealed penetrations. There are penetrations for the feed system, the offgas port/sight glass, and the motor-driven agitator. The feed system consists of a vertical screw to carry plutonium oxide powder, mixed with glass frit, into the melter.
The whole melter assembly fits onto a frame designed to allow for expansion and contraction of the heated materials. Castable refractory slabs provide location for the top and bottom of the vessel. As the melter transitions between room temperature and operation temperature, expansion is accommodated by a spring/slider mechanism that allows the bottom slab to move downward.
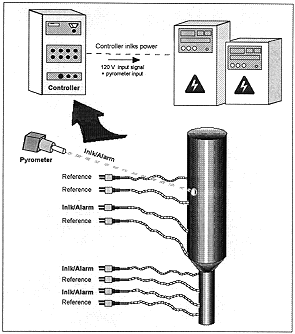
Fig. 2. Temperature control for the induction melter system.
The melter temperature is controlled via a two color optical pyrometer that is focused on the platinum vessel (Figure 2). It has alarm and interlock capability to prevent the vessel from over-heating. Thermocouples are placed along the length of the vessel for reference and for redundant interlocking in case of high temperatures. The drain tube is controlled via thermocouples along its length, with two of them allowing for power interlock.
RESULTS AND DISCUSSION - COMPONENT TESTING
Because of the accelerated schedule for waste form downselection, melter system testing had to be carried out in conjunction with the design of the prototype melter. Testing was done on individual melter components to facilitate data generation. The main melter system components, the melter and induction heating equipment, were tested in a similarly designed system. The drain tube design was tested in a melter system at the SRS, being developed for another DOE program.
Production-scale Melter Testing with Surrogate Plutonium LaBS Glass
The induction melter system that was tested used essentially identical induction system components, namely, induction heating systems for the vessel and drain tube (with similar power supply sizes) from the same vendor chosen for plutonium melter. The melter vessel was constructed from Pt/Rh alloy and had approximately the same volume. The melter also contained an integrated stirrer and utilized the same temperature control strategy as was designed for the plutonium melter.
The main objective of the test was to demonstrate the processability of the LaBS glass by successfully incorporating plutonium surrogate in the LaBS glass on a production-scale. Secondary objectives were to demonstrate safe operation of the melter and to examine operating parameters during processing.
All objectives were met without difficulty. Eight weight percent hafnium oxide, the plutonium oxide surrogate, was fully incorporated in the LaBS system after four hours at 1500°C. During the campaign approximately 20 pounds of glass were produced. No crystallization or undissolved batch was detected in the glass. This test with 8 wt % HfO2 corresponded to a 10.4 wt% PuO2 loading in the glass on a molar basis.
The melter was safely operated, with the same redundant interlocking capabilities as the plutonium melter. A number of parameters were monitored during operation (Figure 3). The melter power and current remained very steady over the six hour operating period. The temperature decreased slightly when feed was added to the melt. However, the temperature quickly rebounded as the feed was incorporated into the melt.
Analysis of the glass samples taken during the melt pour indicated that the glass composition was homogeneous throughout the melter (Table I). It is important to note the uniformity of the hafnium oxide (plutonium surrogate) concentrations in the samples. From nuclear material criticality and accountability standpoints, the uniformity of the hafnium oxide within the melter was especially encouraging. The glass sample concentrations also compared very closely to the expected concentration values (Table II). Very little volatility of the glass components was seen during the testing. This is especially noteworthy for boron which is often seen to volatilize from glass melts. Essentially no deviation was seen in the hafnium oxide concentrations which is again positive from accountability and criticality safety standpoints.
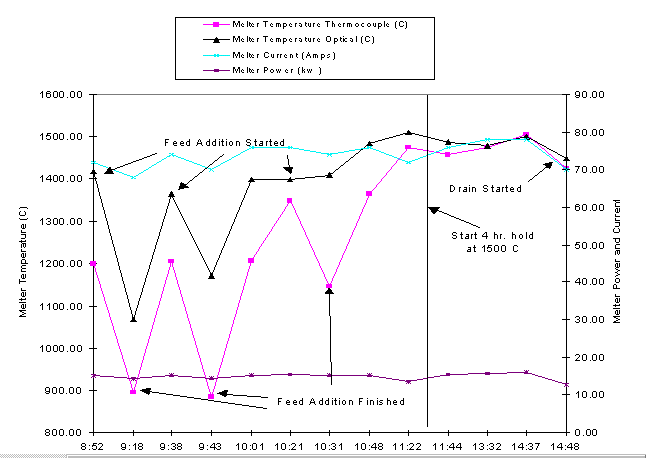
Fig. 3. Melter parameter data from surrogate plutonium LaBS glass campaign.
Table I. Production-scale Melter Sample Data
|
Oxide |
Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
Sample 5 |
|
SiO2 |
28.6 |
28.5 |
28.8 |
28.2 |
29.5 |
|
B2O3 |
10.7 |
10.7 |
10.5 |
10.6 |
10.7 |
|
Al2O3 |
19.4 |
19.6 |
19.3 |
19.2 |
19.3 |
|
SrO |
2.3 |
2.3 |
2.4 |
2.4 |
2.3 |
|
ZrO2 |
1.0 |
1.0 |
1.0 |
1.0 |
0.9 |
|
La2O3 |
11.6 |
11.2 |
11.3 |
11.6 |
10.8 |
|
Nd2O3 |
11.1 |
11.1 |
11.1 |
11.4 |
10.9 |
|
Gd2O3 |
7.4 |
7.3 |
7.5 |
7.4 |
7.4 |
|
HfO2 |
7.9 |
8.2 |
8.0 |
8.1 |
8.0 |
Table II. Glass Sample Data and Expected Compositon
|
Oxide |
Sample Average |
Expected |
Difference |
|
|
SiO2 |
28.74 |
27.83 |
0.9 |
3% |
|
B2O3 |
10.63 |
10.56 |
0.08 |
1% |
|
Al2O3 |
19.35 |
18.76 |
0.59 |
3% |
|
SrO |
2.35 |
2.29 |
0.06 |
3% |
|
ZrO2 |
0.97 |
0.95 |
0.02 |
2% |
|
La2O3 |
11.30 |
10.91 |
0.39 |
4% |
|
Nd2O3 |
11.12 |
11.26 |
-0.13 |
-1% |
|
Gd2O3 |
7.41 |
7.69 |
-0.28 |
-4% |
|
HfO2 |
8.03 |
8.06 |
-0.03 |
-0.4% |
Melter Drain Tube Testing
The second series of testing involved examination of the drain tube performance to be used for the plutonium melter system. The drain tube design had been duplicated from a bushing melter system being developed for another DOE program at SRS [4]. Therefore, the existing bushing melter was provided an adequate test bed to examine the pouring behavior of the LaBS glass. Two drain tube test campaigns were conducted using the LaBS glass with plutonium surrogate. The first test was used to quantify melter pour rates, while the second test examined the starting and stopping behavior of the glass pour.
In the first test, pour rates were determined at periodic intervals as the melter completely drained. A total of ten measurements were made on the roughly 25 pounds of glass produced (Table III). As anticipated, the pour rates dropped as the glass level (i.e. head pressure) in the melter was reduced. The glass flow was no longer a steady stream during the last two measurements. At such low flow rates there is a potential for stringer formation, so in production operations glass flow would be stopped prior to complete draining leaving a small heal of glass in the melter.
In the second test campaign, the pour stream was started and stopped several times during the pours to demonstrate that the air-jet system was adequate for the relatively low-viscosity LaBS glass. Glass flow was started in all cases in less than two minutes (Table IV). When glass flow was stopped by cooling the drain tube with air, minimal dripping and no stringer formation was observed. The glass flow was stopped without difficulty in a matter of seconds (Table IV).
Table III. Glass Flow Rates
|
Sample |
Rate (kg/hr) |
Sample |
Rate (kg/hr) |
|
1 |
12.3 |
6 |
7.7 |
|
2 |
11.8 |
7 |
6.8 |
|
3 |
10.5 |
8 |
6.2 |
|
4 |
9.5 |
9 |
5.4 |
|
5 |
8.6 |
10 |
0.9 |
Table IV. Starting and Stopping Times for Glass Flow
|
Pour Number |
Start Time (sec.) |
Stop Time (sec.) |
|
1 |
95 |
60 |
|
2 |
105 |
35 |
|
3 |
65 |
n/a* |
|
4 |
65 |
31 |
|
5 |
54 |
10 |
|
6 |
70 |
n/a* |
|
7 |
80 |
n/a* |
*
Melter completely drainedCONCLUSIONS
A prototypical induction heated melter, based on commercial technology, was designed and built for the vitrification of surplus weapons-useable plutonium. The melter is capable of operating at the high temperatures necessary to incorporate high concentrations of plutonium oxide in the LaBS glass. The melter incorporates a stirrer to facilitate homogenization of the melt. Feed and off-gas systems are integrated into the design to complete the melter system.
The feasibility of processing production-scale quantities of the LaBS glass was demonstrated by performing melting tests in a melter of similar design. The composition of the resulting glass was essentially identical to the targeted composition. The melter was operated safely and all melter controls performed adequately.
The melter drain tube was demonstrated by performing pour test campaigns on an existing bushing melter with the identical drain tube configuration. These tests demonstrated that effective control of the LaBS glass flow could be achieved with the specified drain tube.
REFERENCES