CERAMIC PROCESS EQUIPMENT FOR THE
IMMOBILIZATION OF PLUTONIUM
William Brummond and Guy Armantrout
Lawrence Livermore National Laboratory
ABSTRACT
Lawrence Livermore National Laboratory is developing a ceramic form for immobilizing excess US plutonium. The process used to produce the ceramic form is similar to the fabrication process used in the production of MOX fuel.
In producing the ceramic form, the uranium and plutonium oxides are first milled to less than 20 microns. The milled actinide powder then goes through a mixing-blending step where the ceramic precursors, made from a mixture of calcined TiO2, Ca(OH)2, HfO2 and GdO3, are blended with the milled actinides. A subsequent granulation step ensures that the powder will flow freely into the press and die set. The pressed ceramic material is then sintered.
The process parameters for the ceramic fabrication steps to make the ceramic form are less demanding than equivalent processing steps for MOX fuel fabrication. As an example, the pressing pressure for MOX is in excess of 137.0 MPa, whereas the pressing pressure for the ceramic form it is only 13.8 MPa. This translates into less die wear for the ceramic material pressing. Similarly, the sintering temperatures and times are also different. MOX is sintered at 1,700° C in 4% H2 for a 24 hour cycle. The ceramic form is sintered at 1350° C in argon or air for a 15 hour cycle.
Lawrence Livermore National Laboratory is demonstrating this ceramic fabrication process with a series of processing validation steps: first, using cerium as a surrogate for the plutonium and uranium, second, using uranium with thorium as the plutonium surrogate, and third, with plutonium.
INTRODUCTION
The US has declared a portion of it's plutonium inventories surplus to defense needs. Unlike surplus highly enriched uranium, plutonium cannot be made unavailable for future weapons applications by simple isotopic dilution. In order to dispose of the surplus plutonium, DOE has selected a dual-track approach involving 1) fabrication of MOX fuel for reactor burning and 2) immobilization of impure plutonium stocks in an acceptable form for geologic repository disposition. The current preferred form for the immobilization of surplus US plutonium are titanate-based minerals containing sufficient neutron absorbers to assure criticality safety. The work to be reported in this paper discusses the fabrication technology for this immobilized form.
The primary process currently under development for the production of the immobilized form is based on the cold press and sinter process. This approach was chosen on the basis of relative simplicity, minimal cost, and the relative maturity of closely related technologies. The most closely related technology at present is that used for the fabrication of mixed oxide reactor fuel. The basic flow diagram for this process is shown in Figure 1.
![]()
Figure 1. Cold Press and Sinter Process
For the first step in this process, plutonium oxide which is received from a conversion process is milled to a particle size of less than 20 microns. Reduction to this particle size is necessary to ensure essentially complete reaction of the plutonium with the ceramic precursors in subsequent sintering operations. Larger particles will only partially react, leaving islands of plutonium-rich minerals or unreacted plutonium oxide encased in the mineral structure. While this may be acceptable for the desired repository performance, it complicates the form characterization and acceptance for the repository if present in significant quantities.
Uranium oxide will also be co-milled or blended in during this step. Uranium is required in about a 2:1 ratio with the plutonium oxide feed to ensure the dominant formation of the pyrochlore mineral phase which is desired. Some of the uranium will come from the impure plutonium feeds. Additional uranium will be added to achieve the 2:1 ratio in the form of U2O3.
Following the milling and blending of the plutonium and uranium feeds, the previously prepared precursor oxide feeds are added to the actinides. This precursor material, which has particle sizes comparable to the actinide oxides (<20 microns) will be uniformly blended with the actinide feeds. This blending must be uniform on a micro-scale to ensure uniform reaction of the actinides and pre-cursors. If this blending is not adequately performed on the micro-scale, then islands of plutonium rich and plutonium lean mineral forms can result. Again, while these non-uniform products may well be acceptable with regard to repository performance, they will complicate the licensing and repository acceptance process.
Blending of the ceramic precursor can be accomplished in several different ways. One way is to use ball-milling with an aqueous vehicle. Good blending has been achieved on the scale necessary to ensure uniform mineral products. However, the addition of water to the process complicates criticality control and necessitates additional processing steps. An alternate technique which is being developed for plant use for ceramic formation is the use of a high energy device such as an attritor mill. The mill provides the additional energy necessary to break up the agglomerated particles of the plutonium and uranium oxide for blending on a micro scale. Current work is ongoing to verify that this is the case for the actinide / precursor system currently employed in the baseline ceramic formation.
Following the blending step, the granulation step is used to convert the micron size powder blended powder to a flowable material to facilitate uniform flow into the press die set. This is necessary since the finely milled blend tends to clump and is difficult to meter and transfer to the press system. In addition, handling of such finely milled oxide presents the possibility of excess dusting when the milled and blended powder is removed form the contained powder preparation unit. Once granulated, the blended milled product is easily transferred, metered, and stored and is relatively dustless during handling.
The next step in the process formation is the pressing of green ceramic discs in preparation for sintering. Since the sintering used for the ceramic formation is reactive sintering, relatively low densities are required for the green pressing operation product. This greatly minimizes die wear and press operation difficulties. However, due to the low pressing force, the resulting green ceramic materials are relatively fragile until they are sintered, and some care should be taken in handling.
The sintering operation is carried out in either an argon or air atmosphere. The temperature of the green ceramic discs are raised at 3° C per minute to reach 300° C where the temperature is held for 2 hours to thoroughly dry the green ceramic and decompose any lubricants or binders. Then the temperature is again ramped at 5° C / min. to 1350° C. The temperature is held at this temperature for 4 hours to sinter the ceramic form. Following this, the ceramic is cooled at < 10° C / min. to near room temperature prior to removal from the furnace. At this point, fabrication of the ceramic is complete and the rugged product can be handled easily for characterization and subsequent disposition operations.
COLD PROCESS DEMONSTRATION
The first step in the development of the plant processing technology is to develop the basic chemical and powder handling processes from bench scale laboratory experiments to large scale operations which can be efficiently used in a plant. Due to the difficulty in working with plutonium, early process development and demonstration used cerium oxide as a surrogate for plutonium oxide. In many milling, handling, and mineral formation operations, we have found excellent similarity between forms produced either with cerium oxide or plutonium oxide.
Early experimental milling operations used a jet mill to size reduce the cerium and plutonium oxides to the required size. The mill functioned well, producing 1.5 to 3 micron material for both cerium and plutonium oxide. This milled feed was used to produce very satisfactory ceramic forms with good reaction uniformity. However, only 85% of the mill feed was recovered in the mill's cyclone separator. The mill was subsequently modified with a second stage of cyclone separation and a HEPA filter. Over 92% of the material was now recovered from the cyclone separators, with the balance entrained in the HEPA filter. This is still too much loss for a plant operation, and other milling techniques are being investigated with a high speed attritor currently being the preferred option.
In the first run of a small attritor such as may be used in a scale-up mode in the plant, manganese oxide was quickly reduced from +200 mesh (seventy five microns) to a relatively uniform powder with particle sizes on the order of one micron. Subsequent milling experiments are underway for all of the constituents used in the ceramic material to verify acceptable mill operation.
A "V" shell blender with an internal "attritor bar" was initially used for the initial blending and granulation studies. Dry blending of the milled actinides and precursors in the V blender using either cerium or plutonium resulted in ceramic products with agglomerated regions of a non-uniform nature which indicated poor mixing on a micro-scale. Clearly, the agglomerated particles of both the milled actinides and precursors have sufficient binding such that the tumbling of the dry powders in the V blender had insufficient energy to break up the agglomerates in a reasonable time for micro-scale blending. The action of the "attritor bar" was also insufficient to achieve the fine blending required. A sample of the V blender material was subsequently dried and sent to an attritor mill company for further blending in an attritor mill in order to further evaluate the possibility of attritor blending as an alternative to wet ball mill blending. The result was an extremely uniform blend which produced a uniform mineral phase in the sintered product.
The V blender was also used for the granulation of the blended mixture. In this case, much better performance was achieved. In operation, polyethylene glycol dissolved in water was used to help granulate the milled oxides during the tumbling operation in the V blender. The resulting granulated powder poured and pressed very well.
The next step in the process involves the pressing of the milled and granulated feed into a form suitable for sintering. Since we are still interested in proof of operation rather than a final plant prototype, we have used a simple single ram hydraulic press to produce the "green" ceramic form. A die set was designed and fabricated which formed an 8.9 cm diameter by 3.8 cm thick puck. This pressed ceramic green form can contain approximately 50g of plutonium and 100g of uranium. During a series of compacting tests using this die set and the ram press, it was determined that 13.8 MPa is sufficient pressure to produce a good product using our current sintering temperature and time. Higher pressures only decrease die life, with no gain in final product density. Oleic acid in acetone was used as a die lubricant. Other compounds will be evaluated.
Following pressing, the green ceramic forms are sintered using the temperature cycle described above. All reactive sintering cycles so far have been performed in a box furnace. The pucks were placed on ceramic "furniture" (Al2O3) with additional granular Al2O3 to keep the green ceramic from sticking during firing. Atmosphere of both argon or air have been used. The total firing time is 15.5 hours. During reactive sintering, the pucks shrink from 8.9 cm diameter to 6.4 cm in diameter and from 3.8 cm thickness to 2.5 cm thickness. We have produced over 800 consistent final ceramic forms based on the use of cerium as a surrogate for both plutonium and uranium without a failure due to fracture (figure 2). On sample runs of these surrogate ceramics where production conditions were controlled, we have consistently obtained final forms with minimal closed porosity and densities equal to 93% of the theoretical density.

Figure 2. We have produced hundreds of ceramic forms without failures
URANIUM AND THORIUM
The second step in this process development is to replace the cerium oxide with uranium oxide and thorium oxide (a better milling surrogate than cerium oxide but still a good chemical surrogate). Additional motivation for developing a uranium capability is the need to incorporate uranium in the ceramic to control the final mineral phase formation. A 30 foot, six workstation fumehood has been installed for uranium and thorium operations. The fumehood (figure 3) was designed with the flexibility to provide for a wide range of developmental equipment. Currently installed equipment includes a jar mill, an attritor mill, a calcining furnace, and a hydraulic press identical to the ones used in the cerium development described above. A dedicated sintering furnace is provided external to the fumehood for uranium ceramic sintering operations. Note that once the powder is pressed, dust control is not an issue for the uranium samples.

Figure 3. Fumehood for uranium and thorium work
Uranium operations were initiated with the forming of uranium bearing ceramic forms but still using cerium as a surrogate for plutonium. These ceramic products were made using a wet ball milled slurry to produce a product which is comparable to the baseline final form in terms of mineralogy and uniformity. Past experience with our all-cerium surrogates has demonstrated that such wet mixed ceramic materials exhibit excellent micro blended characteristics.
Eventual plant processes will utilize dry processes with high energy milling and blending as described above. The necessary uranium-qualified attritor mill has been installed and is undergoing initial milling testing with surrogate materials and the ceramic precursors. As soon as these tests are complete, we will begin production of our uranium surrogates using the high energy attritor mill for all milling and blending operations.
All unit operations which are currently being developed with cerium or uranium will also be demonstrated with plutonium. Identical equipment for granulation, pressing, and sintering has already been installed in gloveboxes in our plutonium facility (to be described below) and are ready for operation. In addition, we earlier installed a jet mill for preparing finely milled plutonium oxide for our early experimental development studies. We are currently designing an attritor mill system for installation later this year which will be used for high energy milling and blending operations identical to those currently being developed and tested with uranium in our newly activated uranium line.
UNIT OPERATIONS AND INTEGRATED SUBSYSTEM TESTING
The final step of our development before deployment will be with plutonium. Initially, we will complete the installation of equipment as indicated above which is identical to that currently installed in our uranium line. After successful demonstration of this equipment, our next work will be the design and demonstration of plant-prototypic processing using plutonium feeds expected in the actual operating plant.. This integrated subsystem will consist of the form-critical operations which are milling, blending, and pressing. Sintering is a well-known operation and does not require prototyping. This system will also be used to answer powder handling, accountability, maintainability and dose issues.
To date a press and die set along with a sintering furnace have been installed into an existing glovebox (figure 4). This equipment is in the shake down mode. The design for an attritor mill and a new glovebox are underway. Installation in the Plutonium Facility is planned for early summer.
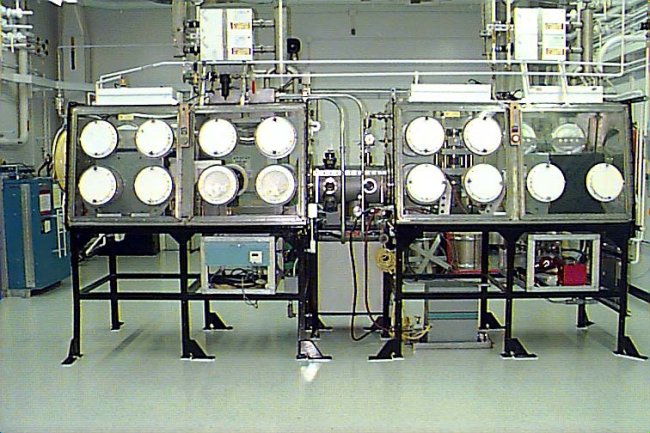
Figure 4. Plutonium pressing and sintering glovebox
Conceptual designs have been developed for the plant prototypic system based on our work to date, and will be finalized pending the confirmatory outcome of our current milling and blending studies with uranium using our new uranium attritor mill system. The design of this system is scheduled to be complete next year, with start-up and confirmatory operation with plutonium schedule for the year 2000.