IMMOBILIZATION FEED STOCKS AND THEIR PREPARATION
FOR IMMOBILIZATION
Mark Bronson, Julio Diaz, Thomas A. Edmunds,
Leonard W. Gray, and David C. Riley
Excess Fissile Materials Disposition Program
Lawrence Livermore National Laboratory
Thomas L Rising
Los Alamos National Laboratory
ABSTRACT
The feed materials projected to be dispositioned by the Fissile Materials Disposition Program (FMDP) contain a broad distribution of plutonium sources, plutonium isotopes, uranium, and a broad spectrum of tramp impurities. The isotope 240Pu is of interest for the purpose of denaturing weapons grade plutonium; 241Am is of particular interest as a source of gamma and neutron dose to the operators. The materials categories include pits and clean metal, impure metal, plutonium alloys, clean oxide, impure oxide, mixed uranium/plutonium oxide, alloy reactor fuel (e.g., the plates from the ZPPR at INEL), and oxide reactor fuels (e.g., the un-irradiated FFTF fuel). In addition, some materials which have been stabilized and treated in some fashion by the EM Program will also come to Immobilization for disposition. These materials will be transformed into plutonium-uranium oxides, and the impurities and the isotopics will be equalized somewhat by blending prior to being fed to one of two possible immobilization forms
The Excess Fissile Materials Disposition Program’s Record of Decision (ROD) published in January of 1997 by DOE/ MD creates a window of opportunity for increased coordination between the EM and MD programs. Some materials that fully comply with STD-3013 will be very difficult to process by either the MOX or the immobilization options. Conversely, some materials that do not satisfy STD-3013 because they are less than 50 wt% Pu or are not in a long term storage container, would make excellent feeds for the immobilization option. Additionally, there are several subsets of materials that will be difficult to process by any of the MD disposition options. These must have further stabilization or conditioning prior to being received by the disposition program. A decision must be made about where and when and by whom this conditioning should occur. The anticipated feed stocks, their isotopics and anticipated impurities, their treatment in the plutonium conversion operations (e.g., decladding to remove the reactor fuel from fuel assemblies, HYDOX for metals alloys, calcination to remove water and carbon, etc.) and their blending for downstream immobilization, all must be considered.
INTRODUCTION
Background
Between 1944 and 1994, the United States used reactors at Hanford and Savannah River to produce 103.4 tonnes of plutonium. Some of this plutonium was burned to make higher isotopes (Am, Cm, Bk, Cf, etc.) and some was used in weapons testing. Three different Programs of the Department of Energy must deal with the safe management and disposition of this plutonium now that the "Cold War" has ended. Defense Programs will maintain a strategic reserve of plutonium for long-term defense needs. That material which is declared excess to programmatic needs will either be disposed of as waste in the Waste Isolation Pilot Plant (WIPP) by Environmental Management Programs (EM) or dispositioned in accordance with the "Spent Fuel Standard" by the Materials Disposition Program (MD).
Environmental Management (EM)
During World War II and the Cold War, the United States developed a massive industrial complex to research, produce, and test nuclear weapons. This complex included nuclear reactors, chemical processing plants for targets and fuels, weapons manufacturing (metal machining) and assembly plants, chemical recycle plants for returned weapons, and laboratories that manufactured tens of thousands of nuclear warheads.
Weapons production stopped in the late 1980s, initially to correct environmental and safety problems, but was later ended indefinitely because of the end of the Cold War. This abrupt halt to nuclear material production facilities left a legacy of plutonium, sufficient to fabricate thousands of nuclear weapons, in a variety of chemical and physical forms, packaging configurations, and geographical locations. These materials must be stabilized, safe-guarded, and dispositioned.
In 1989, the Department of Energy established the Environmental Restoration and Waste Management program, now called the Environmental Management program, to consolidate ongoing activities and accelerate efforts to deal with the inactive production facilities and sites, and the accumulated waste, contamination and the residual backlog of special nuclear materials.
The Environmental Management program encompasses
It is assumed that Environmental Management will dispose its plutonium by one of two paths:
Materials Disposition (MD)
As part of their agreement to make reductions in nuclear weapons, the United States and Russia have undertaken joint programs to develop and implement approaches for ensuring the secure management and eventual disposition of fissile materials rendered surplus by arms reductions. These endeavors have been endorsed with highest priority by the presidents of both countries. To demonstrate the U.S. government’s commitment, President Clinton declared in March, 1995 approximately 50 metric tons (MT), including about 38 MT of weapon-grade material, surplus to U.S. Russia has also indicated that a similar quantity of plutonium will be made surplus.
The responsibility for U. S. fissile materials disposition resides with the Office of Fissile Materials Disposition, created in 1994 to manage DOE activities relating to the management, storage, and disposition of surplus fissile materials. The goal of the plutonium disposition program is to "make the plutonium as unattractive and inaccessible for retrieval and weapons use as the residual plutonium in the spent fuel from commercial reactors." This goal, referred to as the "Spent Fuel Standard," was originally stated in a report by the U.S. National Academy of Sciences (NAS) Committee on International Security and Arms Control(Ref. 1).
MD has been conducting development activities and evaluations of promising disposition technologies leading to a choice of the best technologies for implementation. In the Record of Decision (ROD) for the Storage and Disposition of Weapons-Usable Fissile Materials issued in January 1997 (Ref. 2), DOE announced its decision to pursue two alternative technologies for Pu: (1) irradiation of Pu as mixed-oxide fuel in existing power reactors, and (2) immobilization of Pu into large solid forms containing fission products as a radiation barrier. The immobilization alternative will be used for the disposition of impure Pu materials and could, if desired, be used for the larger quantity of pure Pu materials.
ASSUMPTIONS
1. Neither EM nor MD will retain ownership of the excess plutonium. It is assumed that EM will either prepare the plutonium as necessary to alleviate proliferation concerns and for shipment to WIPP or stabilize the materials as necessary and transfer the materials to MD. It is also assumed that MD will disposition the materials in a fashion that meets the "spent fuel standard."
2. The head-end of the plutonium immobilization facilities will have the capability:
3. The first stage of immobilization will be conversion of plutonium oxide to stable minerals, predominantly pyrochlore, in a multiphase ceramic. The second stage of immobilization will be encasing these ceramic forms in HLW glass at the DWPF.
4. The Immobilization Facility will operate as an unclassified facility with either IAEA or other international bilateral inspections anticipated.
5. All plutonium declared excess (Materials not part of the strategic reserve or scheduled to be transferred to WIPP) will be available to the immobilization facility on demand.
FEED MATERIALS
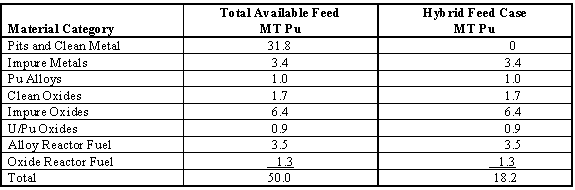
There are a number of ways to sub- divide the anticipated feedstock that is assumed to be coming to immobilization. The EIS divides the feedstock into two cases, a 50 tonne case in which all Pu comes to immobilization and a 18.2 tonne case in which the Pu from clean metal and pits go to MOX feed. These two cases are given in Table I.
Table I. Immobilization Feed Materials

The categories specified in Table 1 cover large amounts of materials with significant variations in properties. These can be subdivided into subcategorize so that the individual sub-categories, which will become feed streams, will have less variation within them.
Current Feed Compositions
The compositional data for many of the current streams is limited because the only data required to be recorded on the streams is the amount of fissile material, for material control and accountability (MC&A) purposes. The available data come in one of four forms: engineered materials data, material specifications, sampling data, and engineering knowledge. The level of knowledge decreases as one moves down the list.
Engineered Materials
Engineered materials are well known because they have been designed to meet certain criteria. The feed streams that fit into this group are pits, the alloy reactor and oxide reactor fuels. With the exception of 241Am, U, and Ga, the tramp impurities in Pits are very low, typically in the <100 ppm range. The alloy reactor fuel is dominated by the ZPPR fuel. ZPPR fuel is of predominately two types: Pu-Al alloy with 1% aluminum and Pu-U-Mo alloy with compositions of 28%:68%:3%; 20%:78%:2%; and 28%:68%:2%. The plutonium isotopic varies from 5 to 27% 240Pu. The total uranium content is 7400 kg. The oxide reactor fuel is dominated by the FFTF fuel. The mixed oxide FFTF fuel was manufactured but not irradiated in a reactor. The plutonium consists of 12 to 26% 240Pu with the total plutonium compositions being 13 to 27% with depleted uranium oxide (4000 kg) being the remainder. Tramp impurities are nominally less than 100 ppm for each impurity element.
Processing Requirements for Engineered Materials
The metals will have to be converted to oxide and the ceramic reactor fuel pellets will have to be ground to size. No other processing is required. This is excellent blend stock for the Immobilization Process.
Specification Materials
The material specification data is given as a range of allowable values for the impurities. Material specification values were available for the clean metal and clean oxide categories. Clean metal generally contains less than 100 ppm of any given chemical impurity. The metal can be either weapon-grade, fuel-grade, or reactor-grade. The specification for the clean oxide is that the impurities are less than 3 wt%.
In general, the U/Pu oxides at Rocky Flats are mixtures of enriched uranium(500 kg) and plutonium oxides and those at Hanford are primarily materials that were being prepared as FFTF fuel, hence high purity PuO2 and UO2 (800 kg). In general the U/Pu oxides are also fairly high purity materials. Although these are not specification materials because of the uranium content, so far as immobilization is concerned, the tramp impurities are of about the same range.
Approximately one tonne of impure metal is listed in the impure metals category not because of tramp impurities but because of the 240Pu content. Otherwise this appears to be very high quality plutonium metal. Another tonne or so of impure metal has been produced at SRS under the present clean-out program. This metal is not being analyzed so it cannot be placed in the clean metal category. There is no reason to believe that this material is grossly contaminated with tramp impurities. In general, these two materials are as pure as the clean oxide; for the purposes of Immobilization processing, the materials can be placed into the clean category or specifications materials.
Processing Requirements for Specification Materials.
The metals will have to be converted to oxide. The oxide may require grinding and / or calcination. Blending to adjust the 240Pu content is required for this material. No other processing is required. This is excellent blend stock for the Immobilization Process.
Sampling Data, and Engineering Knowledge Materials
For residues in general, the only data required was the fissile content for MC&A purposes. Data on tramp impurities was not required and is not recorded. However, a number of samples of various materials have been sampled and analyzed and coupling the sample results with process knowledge, gives fairly good representations of what is contained within the residues.
Data for some of the lower-grade residues comes from sampling of the streams. Some of these data pertain to individual samples, while some of it is a composite of several samples. The composite data generally provide information on maximum, minimum, and average concentrations. There are composite data for the Rocky Flat’s Ash and Ash Heels presented in Reference 5. and in the Rocky Flats EIS. (Ref. 6)
The fourth type of data is based on engineering judgment. The quality of this information is dependent upon the knowledge of the person(s) estimating the values. This type of data was used to estimate the composition of Hanford impure oxide (Ref. 7), DOR residue, ER Residue, MSE Residue (Ref. 8) and fluoride residues.
CONVERSION PROCESSES
The processing to occur in the Pu Conversion portion of the Immobilization Facility is shown in Table II. The Pu Conversion processes are HYDOX conversion of metal to oxide, halide wash, decladding and grinding. The HYDOX process involves hydriding the plutonium metal. The hydride is then converted to an oxide. The HYDOX process is used instead of furnace oxidation because during furnace oxidation a protective coating of oxide forms that slows down the oxidation process significantly. The hydride that forms during hydriding has a significant volume expansion. This breaks any protective coating that might form. Hydriding also hydrides the plutonium and uranium preferentially over other metals. Because the HYDOX process depends on the fact that plutonium reacts with hydrogen to form a hydride, there is a limit to how much impurity metals can be with the plutonium. If the impurity metals do not hydride, they could inhibit the hydriding reaction. The hydriding process has been tested on a number of materials. The largest amount of impurity tested to date was an anode heel that had about 11 wt% gallium. This can be used as a conservative estimate of how much metal impurities can be hydrided. Converting the 11 wt% to atom percent and allowing a margin for uncertainties results in a limit of 27 atom % non-hydridable metal impurities. The relative weight percent of the metal impurity varies depending upon the molecular weight of the metal. For low molecular weight metals, the weight percent is low. For high molecular weight metals, the weight percent is high. The wt% limit for some example metals are: 4 wt% Al, 6 wt% Ca, and 4 wt% Mg.
Table II. Pu Conversion Processes
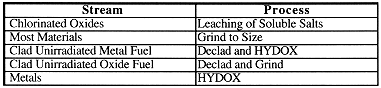
The Pu Conversion front-end of the Immobilization Facility will have a chloride wash station. This equipment is sized to handle the chlorinated oxide that was generated as a result of ER processing at Rocky Flats (Rocky Flats Chlorinated Oxides). It is currently sized too small to handle all of the salts that are present in the Complex. We assume they will be treated as part of the stabilization program.
The Pu Conversion front-end will also declad the oxide and alloy reactor fuels. It will also have size-reduction equipment to break down material sizes to that desired for the first stage immobilization process (~ 10 micron).
CONCLUSIONS
One can divide the Pu feedstock into three groups:
REFERENCES
FOOTNOTE
Work performed under the auspices of the U.S. Department of Energy by the Lawrence Livermore National Laboratory under contract number W-7405-ENG-48.