SALT PROCESS CELL FLOWSHEET MODIFICATIONS TO
MINIMIZE WATER AND COPPER ADDED TO HIGH
LEVEL WASTE SLURRY IN THE DEFENSE
WASTE PROCESSING FACILITY
C. S. Boley, D. P. Lambert, R. A. Jacobs
Savannah River Technology Center
K. M. Willoughby
L.G. Rich Environmental Research Laboratory, Clemson University
ABSTRACT
Flowsheet modifications that have been made to chemical processing in the Defense Waste Processing Facility (DWPF) at the Savannah River Site will minimize the addition of water to the waste streams prior to vitrification. The modifications increase the production rate of the High Level (radioactive) Waste (HLW) by shortening the DWPF batch cycle time, decrease the high boiling organic (unwanted in the product) concentration of the Precipitate Hydrolysis Aqueous (PHA) product, decrease the copper catalyst added, and increase the hydrolysis reaction rate. The new process will lead to higher hydrogen production that must be handled to ensure a flammable mixture is not formed.
BACKGROUND
The Defense Waste Processing Facility (DWPF) at the Savannah River Site is a waste vitrification facility designed to immobilize high-level radioactive isotopes in borosilicate glass. The radionuclides are composed primarily of uranium, plutonium and cesium-137 but include strontium-90, as well as other trace fission products, currently stored as salt cake and sludge in underground tanks. The facility is composed of three main processing elements: a Salt Cell, a Chemical Cell, and a Glass Melter Cell. This paper will deal with laboratory-scale work performed in support of operation of the Salt Processing Cell (SPC).
The salt cake is treated with sodium tetraphenylborate to precipitate cesium-137 from the tank farm salt waste and with monosodium titanate to adsorb the strontium-90 and uranium and plutonium isotopes. An insoluble tetraphenylborate salt is formed with cesium-137, as well as with potassium and ammonium ions present in the waste. The insoluble tetraphenylborate salts are fed to the Precipitate Reactor (PR) in the DWPF Salt Processing Cell for acid hydrolysis and removal of organic compounds.
Prior to feeding, formic acid and copper catalyst (copper nitrate) are added to the reactor heel. The reactor is heated to 90 degrees and the slurry is fed. Benzene vapor evolves as the tetraphenylborate in the slurry undergoes a stepwise decomposition, is condensed and collects above a water layer in the Precipitate Reactor Condenser Decanter (PRCD). After the feed period, the vessel is held at 90 degrees for five hours to ensure the destruction of phenylboronic acid (PBA, the last step in the tetraphenylborate composition) and diphenyl mercury (which must decompose to elemental mercury). The vessel is then heated to boiling to steam strip the high boiling organics that have formed as a result of benzene radical reactions. The condensate passes through the benzene layer of the PRCD and organic compounds are extracted. The resulting aqueous product (PHA) in the PR is then transferred to the Chemical Processing Cell for concentration and combination with sludge and frit (ground borosilicate glass) prior to transfer to the glass melter for vitrification.
The main vessel in the SPC is the Precipitate Reactor (PR), where the hydrolysis reaction takes place. No processing occurs in the PR unless its steam coils are covered to prevent thermal decomposition of tetraphenylborate and the potential for formation of high boiling organic compounds. These compounds present fouling problems in the vessels and, when in the presence of nitric acid (used in the Chemical Cell), form explosive materials that tend to accumulate in the offgas system. Therefore, the original Salt Cell Process left some PHA behind in the PR to cover the coils. When the hydroxylamine nitrate (HAN) process [1] was implemented to mitigate the impact of high nitrite in the precipitate feed (0.2 g-mol/L), an accumulation of high boiling organic compounds was observed in the PHA from run to run. The process was therefore changed so that all PHA product was pumped out of the PR. However, this required an addition of 5300 - 5700 liters of water to cover the coils before the start of the next run. In 1992, the HAN process was replaced by the Late Wash process [2]. This new process limited the concentration of nitrite in the precipitate feed to <0.01 g-mol/L and consequently reduced the concentration of high boiling organic compounds in the PHA. Pumping out the PR completely became unnecessary. However, it remained part of the process throughout Waste Qualification Runs.
In 1993, a task team met to discuss methods to reduce the amount of condensate recycle in DWPF. A report was issued recommending, among other things, a return to the original strategy (leaving a heel behind in the PR) [3]. DWPF subsequently requested development work to allow a heel to be left behind [4].
Included in this request was the verification that the reduced copper level (475 instead of 950 mg/kg) was adequate to destroy PBA during hydrolysis, in response to an SRTC recommendation to lower the copper limit in the melter feed [5]. The copper limit was imposed to minimize copper deposition in the melter, thereby lengthening melter life.
SUMMARY
A modification to the Precipitate Hydrolysis flowsheet for DWPF has been developed. Rather than transferring essentially all the aqueous product (PHA) to the Precipitate Reactor Bottoms Tank (PRBT) at the conclusion of each Precipitate Reactor (PR) cycle (necessitating the addition of large amounts of water to the PR to ensure the steam coils are covered before commencing the next PR cycle), this proposed flowsheet leaves behind sufficient PHA to assure the steam coils are covered in the subsequent batch. This Large PHA heel flowsheet (which leaves 5700 liters of PHA behind as opposed to the Small PHA heel of approximately 100 liters) eliminates the need for water addition and consequently produces an aqueous product significantly higher in total solids (soluble + insoluble) concentration. The advantages of this process are:
Based on the testing described in this document, it is recommended that the copper level in the PR be changed to 475 mg/kg and that a PHA heel of 5,700 gallons be left behind in the PR after each batch. This heel will result in a new PHA steady state concentration of 5.53 wt% total solids as opposed to the old level of 4.0 wt%. This increase in solids loading will decrease the PHA batch size by approximately 30% and will allow the PRBT to hold enough PHA for a single SRAT batch (less total PHA will have to be fed since it is at a higher concentration). This will represent a time savings to DWPF of approximately 11-13 hours of SRAT processing. The lower copper level will result in a raw materials cost savings to DWPF, since only 40% of the original amount of cupric nitrate will be used in the process and will essentially eliminate copper being the PHA batching constraint for glass formulation.
During this testing, the total amount of organic in the PHA product was found to be 1% lower in the large PHA heel process. At the 5 % level, only three streams were found to have a statistically significant amount of organic in the product PHA. The mean differences for these three data streams were 0.340 kgs, 1.247 kgs and -0.133 lbs, respectively. Such slight differences will not have practical significance for DWPF processing. The least significant differences for these results also indicate that the sensitivity of the statistical methods used were sufficient to recognize differences of practical concern. No new high boiling organic compounds were observed in the large PHA heel process product.
During the initial series of large PHA heel hydrolysis process simulations, the peak hydrogen rate increased with each subsequent run (a 1.5 fold increase over the six runs). See Table I. This same behavior was observed during a series of Small PHA heel hydrolysis process simulations (see Table I). Suspecting that PHA solids may have been accumulating in the reactor during pumpout at the conclusion of each run (where the PHA level fell below the agitator during the pumpout), an additional series of hydrolysis simulation runs were conducted where the PHA level in the reactor was not permitted to fall below the agitator. In this series of experiments the maximum hydrogen generation rate observed actually occurred in the second run. Over the seven runs the peak hydrogen rate averaged 0.012 +/- 0.002 kg/hr (based on a 22,700 liter DWPF SRAT batch). These rates were comparable to those seen in similar experiments performed with precipitate from the same batch [6].
Table I. Maximum Hydrogen Flow Comparison, kg/hr/22,700 liter
DWPF SRAT Batch.
|
Run Description |
Run 1 |
Run 6 |
|
Small PHA Heel |
0.0091 |
0.014 |
|
Large PHA Heel |
0.013 |
0.018 |
The important conclusion from these additional tests is that the ability to agitate the contents of the PR during the transfer to the PRBT is crucial to maintaining a safe level of hydrogen production in the Salt Cell in DWPF. If the liquid level in the tank drops below 4500 liters during the transfer, insoluble solids will accumulate and cause an increase in hydrogen production for subsequent batches in DWPF. Therefore it is recommended that the PR level not drop below 5000 liters, so that the vessel will be adequately agitated at all times.
INTRODUCTION
To leave a large PHA heel behind in the PR, it was necessary to demonstrate that no additional amounts of high boiling organic compounds were produced, and that the peak hydrogen production in the in the Salt Processing Cell (SPC) would be within the current DWPF safety limits [7]. To reduce the copper level in the PHA, it was necessary to confirm that the phenylboronic acid (PBA) could be destroyed at a lower copper concentration during the five hour hold time at 363°K. Phase I scoping studies were designed to determine the minimum level of copper that would meet this objective. Phase 2 studies were designed as a process demonstration for both a large PHA heel and a small PHA heel flowsheet, so that the organic accumulation and offgas production in each could be compared. Specific objectives of these tasks were:
Phase I - Scoping Experiments
Phase II - Process Demonstration
If the large PHA heel product contains a significantly higher quantity of high boiling organic compounds, run a SRAT/SME cycle to determine the effects of this PHA on Chemical Processing Cell (CPC) processing. The revised flowsheet must meet all current DWPF requirements including a hydrogen generation rate below the existing hydrogen evolution design basis for the SRAT (0.29 kg/hr for a 22,700 liter batch) and the SME (0.10 kg/hr for a 22,700 liter batch).
DISCUSSION
The current hydrolysis process requires pumping virtually all of the PHA product into the Precipitate Reactor Bottoms Tank (PRBT) after conclusion of the PR cycle, leaving behind a small PHA heel of approximately 100 liters. It also requires the addition of approximately 5,700 liters of water to the PR before every batch to bring the level up to cover the steam coils prior to feeding the TPB slurry. In the large PHA Heel Flowsheet, 5,700 liters of PHA is instead left behind in the PR to cover the coils.
There are several benefits to the large PHA Heel process. Leaving a PHA heel behind will ultimately create an aqueous product higher in wt% solids. This will not only reduce the feed time and shorten the CPC processing time, but will also reduce overall DWPF condensate production. With the new restraint on the amount of copper in the glass (0.35 wt% vs. 0.50 wt%) [8], this flowsheet testing was also designed to verify that the lower copper level (475 mg/kg from 950 mg/kg) is adequate to destroy the PBA. A reduction in the copper level in the PHA will reduce raw materials cost since a simulated heel will not have to be added before each batch. In addition, lower copper levels in the melter could lengthen the melter life expectancy.
EXPERIMENTATION
Method
The Phase I scoping experiments consisted of approximately 2 ½ hours of precipitate feed time followed by a five hour hold period at 363°K. Samples were pulled every hour during the five hour hold and analyzed for PBA in order to determine the reaction kinetics. At the end of the five hour hold, the experiment was concluded. No offgas data was taken during these experiments, because the goal of these experiments was only to establish that the kinetics at a minimal copper level were sufficient to destroy the PBA by the end of the five hour hold.
The Phase II process demonstration experiments consisted of an entire PR cycle. For each process, six sequential runs were performed without cleaning the kettle between runs. Stainless steel kettles were used instead of glass kettles in order to be more prototypic of DWPF. The runs included a 2 ½ hour precipitate feed addition and a five hour hold at 363°K, in addition to a five hour aqueous boil time (to steam strip high boiling organic compounds). Offgas composition (Ar, H2, CO2, N2O) was monitored throughout the run. Argon was used as an internal standard to calculate the outlet offgas flow. Samples were pulled every hour during the five-hour hold and analyzed for PBA concentration to ensure the reaction reached completion in each run.
As will be described in the results section, there was a need to complete seven additional experiments after the process demonstration experiments. Seven sequential runs were performed in a 1L Hastelloy™ kettle without cleaning the kettle between runs. A combined PHA from the six large PHA heel process demonstration experiments was used as the heel in the initial run. These experiments were run exactly like the large PHA heel process demonstration runs, only at ¼ scale. They included a feed time, 363°K hold time and boiling time. Offgas was monitored using Argon as an internal standard. Because offgas production was the only concern, no samples of the reactor contents were pulled during these runs (except at the end of processing).
Precipitate Feed Composition
A single container of late-washed KTPB simulant, irradiated to 200 Mrad and containing ~10.2 wt% total solids was the source of feed used in all experiments. The precipitate feed was from batch PF-143. Key component levels are shown in Table II. This precipitate was chosen as a worst case scenario for the production of high boiling organic compounds. The 40-L carboy containing the irradiated KTPB slurry was spiked with 6,000 mg/kg sludge solids with conservative levels of noble metals to simulate a worst case H2 production. Table III shows the noble metals concentration in the sludge added to the precipitate at 6,000 mg/kg. The HM levels (DWPF design basis) are given for comparison.
Table II- Precipitate Feed Composition
|
Component |
Concentration |
|
Nitrite |
410 mg/L |
|
Cs |
345 mg/kg |
|
Sludge |
6000 mg/kg |
|
Hg |
2340 mg/kg |
|
TPB- |
0.25 g-mol/L |
Table III- Noble Metal Levels in Added Sludge
|
Noble Metal |
Testing |
HM levels |
|
Ruthenium |
0.216% |
0.217% |
|
Rhodium |
0.044% |
0.038% |
|
Palladium |
0.095% |
0.079% |
Scaling
The experiments were designed to be as prototypic of DWPF future processing as possible in 1/7,570 and 1/30,300 scale laboratory facilities. The feed flow rate, boilup rate, and purge rates were all scaled from actual DWPF rates. The scaling for both Phase I & II runs is summarized in Table IV.
Table IV-Scaling for Large PHA Heel Experiments
|
DWPF |
Phase I |
Phase II |
|
|
TPB, total wt% |
8.45% |
8.45% |
8.45% |
|
Precipitate : Heel Volume Ratio |
3.33 |
3.33 |
3.33 |
|
Heel Volume |
5,700 L |
188 ml |
750 ml |
|
Precipitate Volume |
19,000 L |
625 ml |
2,500 ml |
|
Sampling PRFT Prime Water |
170 L |
5.6 ml |
22.5 ml |
|
Transfer PRFT Prime Water |
170 L |
5.6 ml |
22.5 ml |
|
Precipitate feedrate |
114 L/min |
3.8 ml/min |
15.0 ml/min |
|
Batch size |
24,030 L |
793 ml |
3,174 ml |
|
Sampling PR Heel Prime Water |
170 L |
5.6 ml |
22.5 ml |
|
Sampling PHA Prime Water |
340 L |
11.3 ml |
45.0 ml |
|
Transfer PHA Prime Water |
170 L |
5.6 ml |
22.5 ml |
|
CO2 purge rate |
133 sL/min |
4.00 scc/min |
15.82 scc/min |
|
Argon purge rate |
--------- |
0.40 scc/min |
1.76 scc/min |
|
Total purge rate |
133 sL/min |
4.40 scc/min |
17.58 scc/min |
|
Steam flow |
907 kg/hr |
0.50 g/min |
2.00 g/min |
|
Condenser exit temp, °K |
313-318 |
313-318 |
313-318 |
|
SCVC exit temp, °K |
285 |
285 |
285 |
|
Copper nitrate solution, wt% Cu |
1.5% |
1.5% |
1.5% |
|
formic acid , wt% |
90% |
90% |
90% |
Batching
In each of the runs, the feed to heel volumetric ratio was 3.33, scaled down from a 18,900 liter precipitate batch in DWPF. For the small PHA heel runs and the first large PHA heel run, the ~750 ml of heel solution consisted of a 0.25 g-mol/L formic, 475 mg/kg copper solution. In runs 2-6 of the large PHA heel experiments, the 750 ml heel consisted of PHA from the previous run. A 1.47 wt% copper solution and 90 wt% formic acid were added to the heel to target a final product with 475 mg/kg copper and a formic acid molarity of 0.25 g-mol/L. Acid requirements were calculated based on the results of five analyses, including TPB content, an acid titration to pH 5.5, total solids, specific gravity, and nitrite content.
RESULTS
Scoping Runs
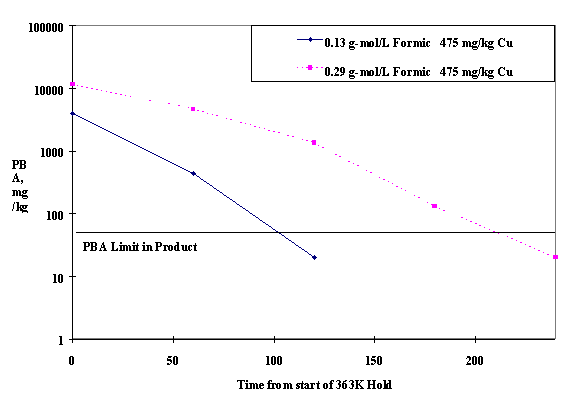
Scoping runs were performed at differing acid and copper levels to determine the minimum copper required (within a range of 475 to 950 mg/kg) to destroy the phenylboronic acid (PBA). These experiments consisted only of a feed cycle and a five hour hold at 363°K degrees. Samples were pulled every hour during the five hour hold and analyzed for PBA concentration. Two experiments were run at 475 mg/kg copper, one with a final acid target of 0.19 g-mol/L and the second with a target of 0.31 g-mol/L. The measured heel acid for the runs was 0.13 g-mol/L and 0.29 g-mol/L, respectively. The lower amount was attributed to minor computational effects (i.e. analysis were low). These runs served to slightly adjust the algorithm used to calculate the acid additions in the larger scale runs.
Figure 1 shows the destruction of PBA over time during both of the runs. The 475 mg/kg copper level was sufficient to destroy the PBA within a five hour 363deg;K hold in both of these experiments. The higher acid run destroyed the PBA at a slower rate than the lower acid run. Previous research shows that there is a parabolic relationship between acid level and PBA destruction rate [9]. The higher level of acid fell on the downward slope of this curve. Because the lower copper level was successful at both acid levels, no other scoping runs were judged required, and this copper level became the basis for the study.

Fig. 1- PBA Destruction During Scoping Runs
Process Demonstration Experiments
A series of six sequential experiments was run for each of two processes (large and small PHA heel). One set simulated the current DWPF Salt Cell process, using a formic acid/copper solution as the heel, and the other set simulated the proposed process, using PHA product as the heel. The entire hydrolysis process was simulated, including the five-hour aqueous boil. Table V shows the average values of what was added and what was produced during the 12 experiments. The low standard deviation values imply high quality control of the experiments.
Table V- Run Average Mass Balance
|
Average |
Std. Dev. |
|
|
Precipitate Mass |
2502 g |
4 |
|
Water Mass |
113 g |
0.2 |
|
Heel Mass |
751 g |
0.04 |
|
Formic Acid Mass |
78 g |
6 |
|
Copper solution Mass |
83 g |
1 |
|
Decanter Aqueous Mass |
79 g |
3 |
|
Decanter Organic Mass |
67 g |
0.2 |
|
Total Mass at Start |
3673 g |
36 |
|
PHA Mass |
3197 g |
18 |
|
Samples Mass |
58 g |
6 |
|
Organic Mass |
273 g |
3 |
|
Aqueous Mass |
94 g |
6 |
|
Total Mass at End |
3609 g |
44 |
|
Total Delta Mass |
-64 g |
13 |
|
Organic Mass Produced |
192 g |
3 |
Copper and Acidity Targets
A 0.25 g-mol/L formic acid concentration in the PHA product was targeted in both processing scenarios. Table VI lists the actual acidity of the final product in each of the six runs. The final acid fell short of the target in both of the initial runs. This was most likely due to an error in one of the analyses or the batching calculation not accounting for some other source of acid loss. After the initial runs, the batching calculation was modified and the target was hit. Due to a miscalculation, a lower amount of formic acid was added in runs 4 and 4H, resulting in the lower product acids.
Table VI - PHA Final Acidity and Soluble Copper Level
|
Run |
Acidity, g-mol/L |
Soluble Copper Level, mg/kg |
||
|
|
Large PHA Heel |
Small PHA Heel |
Large PHA Heel |
Small PHA Heel |
|
1 |
0.19 |
0.18 |
252 |
187 |
|
2 |
0.24 |
0.24 |
207 |
134 |
|
3 |
0.27 |
0.26 |
144 |
115 |
|
4 |
0.22 |
0.21 |
128 |
101 |
|
5 |
0.24 |
0.24 |
126 |
69 |
|
6 |
0.25 |
0.25 |
127 |
104 |
The addition of the 1.47 wt% copper solution targeted a 475 mg/kg copper PHA product (assuming 475 mg/kg soluble copper in the heel). Table VII lists the soluble copper level in the PHA product for each of the 12 runs. For each process, the soluble copper level in the PHA falls with each subsequent run until an apparent steady state value is reached. Cu+2 (soluble) is known to form Cu2O (insoluble) during the five hour 363°K hold time, causing a decrease in the soluble copper by the end of the run. In the large PHA heel process, the use of a heel with a diminished soluble copper level would lead to a steady state final soluble copper level less than 475 mg/kg. In the small PHA heel runs, the copper level should remain consistent (since the heel copper level should remain constant). However, note that the trend for the small PHA heel runs is almost identical to that of the large PHA heel, indicating some effect of the back to back runs on the steady state insoluble copper level.
Table VII - Cu Distribution in Final Product
|
Run |
Cu0 |
Cu+1 |
Cu+2 |
|
|
mg/L |
mg/L |
mg/L |
|
6 |
593 |
10 |
77 |
|
6H |
540 |
10 |
88 |
PHA samples from runs 6 and 6H were analyzed for total copper, soluble copper, and Cu+1. The total copper results were determined by ICP-ES after microwave digestion. The Cu+1 was measured by spectrophotomic absorbance at 454 nm after reacting with bathocuproin. Table VIII shows the calculated distribution of Cu0, Cu+1, and Cu+2. By the sixth run, most of the copper is insoluble. Of the soluble copper, much more Cu+2 is present than Cu+1. It is apparent that the copper is being reduced during processing, although this did not seem to affect the rate of phenylboronic acid destruction.
Table VIII - PBA Destruction Rate Constants, hr-1
|
Run |
Small PHA Heel |
Large PHA Heel |
|
1 |
1.52 |
2.15 |
|
2 |
1.57 |
2.05 |
|
3 |
1.37 |
2.40 |
|
4 |
1.39 |
3.52 |
|
5 |
1.63 |
1.92 |
|
6 |
1.83 |
1.81 |
|
Average |
x |
x |
PBA Kinetics
Samples were pulled every hour during the 363°K hold and analyzed for phenylboronic acid (PBA) using a liquid chromatograph. Table IX lists the PBA destruction rate constants for each of the runs in hr -1. All of the runs were successful in destroying the PBA in less than five hours. The diphenyl mercury was also destroyed well within the five hour time period.
Table IX- Total wt% Solids in PHA Product
|
Run |
Small PHA Heel |
Large PHA Heel |
|
1 |
4.20 |
4.31 |
|
2 |
4.31 |
5.15 |
|
3 |
4.26 |
5.44 |
|
4 |
4.18 |
5.38 |
|
5 |
4.30 |
5.48 |
|
6 |
4.34 |
5.53 |
|
Delta |
0.14 |
1.22 |
Run sequence does not seem to have an effect on the reaction speed, but the recycled heel process destroyed the PBA faster than the current DWPF process (despite the lower soluble copper level). All of the runs destroyed the PBA in less than three hours. The diphenyl mercury was also destroyed in less than 5 hours. It should be noted that the precipitate for each run was isolated separately before the runs started. This was done for four runs at a time (two for each process). If the runs are looked at in blocks of two, the reaction rates look almost identical for each process (i.e., run 1 and 2 are the same, and run 1H and 2H are also the same). This is true for the middle and final sets of runs as well, as a result of similar solids concentration within the sets.
Final Product Solids
Table X lists the total weight percent solids concentration of the PHA product in each of the twelve runs. The large PHA heel product led to a much more concentrated product by the end of six runs. Approximately 99.99% of the steady state solids concentration has been reached by the sixth run. At these concentrations, 32,600 liters of the large PHA heel product would feed the same amount of solids to the SRAT as 41,600 liters of the current DWPF product. This should represent a significant time savings to DWPF.
PRCD Efficiency and Organic Distribution
Samples of the decanter organic and aqueous and the PHA product were analyzed for high boiling organic content. Table X compares the total amount of organic found in the PHA product and the aqueous decanter layers. The organic concentration in the PHA product seems to remain constant over time in both the current process and the large PHA heel process.
Table X - Organic Amounts in Product Streams, kg DWPF scale
|
Decanter Organic |
Decanter Aqueous |
Product (PHA) |
||||
|
Run Number |
Small PHA Heel |
Large PHA Heel |
Small PHA Heel |
Large PHA Heel |
Small PHA Heel |
Large PHA Heel |
|
1 |
21.5 |
21.1 |
2.0 |
2.3 |
32.7 |
32.0 |
|
2 |
94.9 |
95.0 |
2.2 |
2.1 |
32.9 |
34.3 |
|
3 |
135.0 |
123.4 |
1.9 |
2.0 |
32.6 |
33.2 |
|
4 |
215.6 |
192.2 |
2.0 |
2.0 |
35.5 |
33.2 |
|
5 |
189.1 |
217.6 |
2.1 |
2.0 |
34.5 |
34.2 |
|
6 |
199.0 |
260.5 |
2.2 |
2.3 |
38.1 |
36.4 |
Table X also shows the total amount of organic found in the decanter organic layer as a function of each successive hydrolysis cycle. As expected, the high boiling organic compounds accumulate in the decanter organic over time. The organic layer, as would be expected, has a significantly higher amount of high boiling organic compounds than the PHA product. However, in the organic layer the total amount continues to increase from run to run. As a qualitative measure, this pattern demonstrates the efficiency of the decanter. However, the decanter was not scaled by cross sectional area (which would have required a diameter of 1.6 mm), so a comparison to the DWPF decanters is unrealistic.
To compare the organic distribution in both runs, a paired t-test was performed for each high boiling organic compound measured in each product stream (total grams in the PHA, PRCD organic, and PRCD aqueous) over the six runs. There were no statistical differences at the 5% level in the absolute amount of organic in any of the streams except for the product levels of diphenylamine, phenol, and m-terphenyl. The mean differences for these three data streams were 0.340 kgs, 1.247 kgs and -0.133 lbs, respectively. Such small differences will not have a practical significance for DWPF processing. The least significant differences for the results also indicate that the sensitivity of the statistical methods used were sufficient to recognize differences of practical concern. Overall, the large PHA heel process produced 1% less total organic mass in the PHA as observed in these studies.
Offgas
A gas chromatograph monitored the generation of hydrogen throughout the runs. The total outlet flow was calculated using the argon internal standard, and offgas production was scaled to a 22,700 liter PR batch in DWPF. Figures 2 and 3 show the hydrogen generation rates in the small PHA heel and large PHA heel process runs respectively. Sister runs (same sequence number, "H" representing the large PHA heel process) are represented by the same color with the large PHA heel run having a dotted line ( i.e. Run 1 - small PHA heel, run 1H - large PHA heel). The right y-axis represents the raw volume % as measured by the GC. The left y-axis represents the mass flow scaled to a 17,000 liter DWPF precipitate run. Time zero on the x-axis designates the beginning of the five hour 363°K hold time in each run. The times were "adjusted" by ratio so that all of the holds (363°K and boiling) are exactly five hours and all of the heat up times from 363°K to boiling are 36 minutes.
Figure 2 shows the hydrogen generation rate during the small PHA heel runs as a function of time into the hydrolysis cycle. In each run, the rate of hydrogen generation increases during the feed cycle as would be expected. During the five-hour hold period at 363°K, the rate of hydrogen generation peaks in each run and then begins to gradually fall off. With the onset of boiling, the rate of hydrogen generation increases quite rapidly, peaking early into the boil cycle and then with the exception of Run 6 gradually declines. In Run 6 the rate of hydrogen generation appears to begin to increase again after about 4 hours into the five-hour boil cycle. Of particular significance is the tendency for the peak hydrogen rate to be higher with each successive run. As seen in Figure 3, this tendency is even more pronounced in the large PHA heel process simulations.
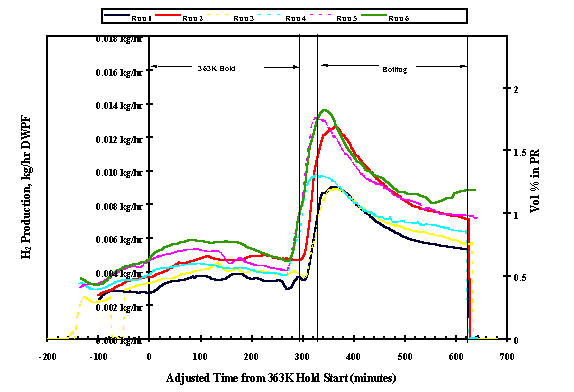
Fig. 2 - Small PHA Heel Process Hydrogen Generation.
Figure 3 illustrates the stairstep effect of hydrogen generation during each sequential run. The last three large PHA heel runs show a significant second peak during the five-hour boil, which gets higher for each sequential run. Both the last two runs (5H and 6H) exhibited a second H2 peak (even greater than that observed at the onset of boiling), nearly reaching a H2 concentration of 2.5 volume % in the PR. Even in runs 3H and 4H, the rate of hydrogen generation has begun to increase before the end of the five-hour boil.
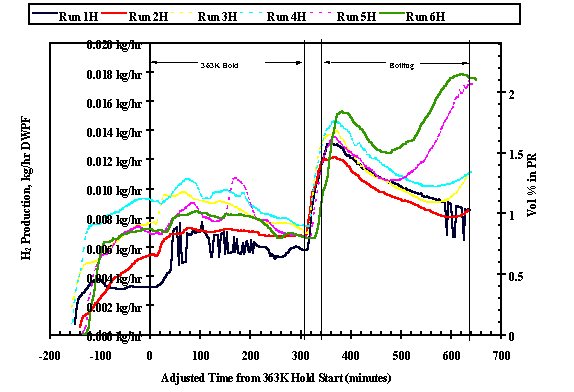
Fig. 3 - Large PHA Heel Process Hydrogen Generation
Explanation of Increasing Hydrogen
Several explanations were considered for the sequential increase in the rate of hydrogen generation. The total precipitate solids concentration in each run increased from 10.2% to about 10.9%, due to evaporation, so more solids were fed in each subsequent run. However, the slight concentration increase (6.4%) was not enough to explain the 108% increase in the hydrogen peak for the large PHA heel runs. An accumulation in noble metals during the runs was considered a more likely explanation. Three possible mechanisms for the accumulation of noble metals in the reactor were considered. First, some fraction of the noble metals in each batch may have plated out on the surface of the reactor. The second postulated mechanism was predicated on the fact that at the end of each run, the entire contents of the reactor were pumped out to a carboy. Although the agitator was left on throughout the transfer, the PHA level falls below the level of the agitator before the transfer is complete. During this final stage of the transfer, some solids could have accumulated in the bottom of the reactor.
The third possible mechanism also was predicated on the experimental procedure for batching the PHA heel for the subsequent run. As previously noted, all the PHA was transferred out of the reactor at the conclusion of the run. Then, a portion of the PHA in the carboy was transferred back into the reactor to serves as a heel for the next run. Since the contents of the carboy were not continuously agitated, a greater solids content in the PHA heel in the subsequent run may have occurred if some settling of solids had occurred in the carboy and the transfer pump suction leg had been at the bottom of the carboy.
To investigate these theories, a number of additional experiments were run. In each of five runs, either a 0.25 g-mol/L formic/475 mg/kg copper solution or the large PHA heel product (combination of all the runs) was boiled for five hours while the offgas was monitored.
To establish a baseline, the formic acid/copper solution was boiled in a clean glass kettle. Minimal hydrogen was measured. The next experiment boiled PHA in the dirty kettle used during the large PHA heel process runs. A large peak was observed at boiling, and the level dropped consistently over the five hours. The third experiment boiled PHA in a clean glass kettle. The peak for the PHA in the stainless kettle was higher at boiling, but the average generation over the last 200 minutes for the runs was identical. These runs presented strong evidence that the PHA solids are responsible for the hydrogen generation.
The fourth experiment boiled the formic acid/copper solution in the dirty kettle used in the small PHA heel process runs. The amount of hydrogen generated in this run was significantly higher than that of the same solution boiled in the clean glass kettle. Because the magnitude of difference was so large, another experiment was done after rinsing the kettle as thoroughly as possible with water. A large amount of solids was noted in the wash water form the kettle. In the last experiment, where formic acid/copper was boiled in the rinsed stainless kettle, a lower amount of hydrogen was measured, although still much higher that that in the clean glass kettle.
The results from these five experiments strongly supported the postulated mechanism that the noble metals (i. e. sludge solids) content of the PHA heel in each successive run was increasing.
As previously described, PHA was not being agitated during the transfer of the PHA from the reactor to the carboy when the liquid level dropped below the agitator, which led to a conclusion that some noble metals and other insoluble species were left behind during the transfer. A second series of large PHA heel experiments was completed in which the PHA was not completely pumped out of the kettle and agitation was maintained throughout the transfer. A minimum of three additional experiments were planned. As a result of problems in experiments 2 and 3, a total of seven experiments were completed. These experiments were conducted in a 1 liter kettle to enable conducting the necessary experiments with the small amount of precipitate feed remaining. Each experiment included approximately 2.5 hours of feeding, five hours of 363°K hold, and five hours of aqueous boil.
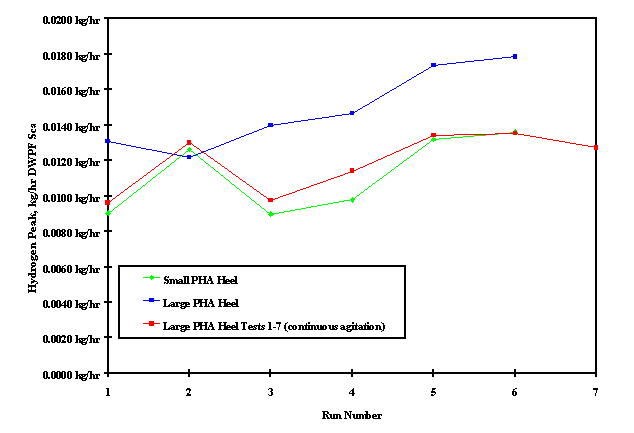
The hydrogen generation rate in each of these seven tests (designated as Test 1, 2 ...7) is summarized in Table XII. The peak hydrogen rates are based on a 22,700 liter batch. The important observations to be made from these seven tests are (1) the absence of increasingly higher peak hydrogen generation rate with each successive test (lending credibility to the second postulated mechanism) and (2) the absence of a second hydrogen peak during the boil cycle which was observed in the last two large PHA heel experiments in Phase II.
Table XI - Hydrogen Peak Comparison
|
New Runs |
Hydrogen Peak, |
Original Phase II Runs |
Hydrogen Peak, |
|
Test 1 |
0.0096 |
Large PHA Heel 1 |
0.0131 |
|
Test 2 |
0.0130 |
Large PHA Heel 2 |
0.0122 |
|
Test 3 |
0.0098 |
Large PHA Heel 3 |
0.0140 |
|
Test 4 |
0.0114 |
Large PHA Heel 4 |
0.0147 |
|
Test 5 |
0.0134 |
Large PHA Heel 5 |
0.0174 |
|
Test 6 |
0.0135 |
Large PHA Heel 6 |
0.0179 |
|
Test 7 |
0.0127 |
x |
x |
Figure 4 shows the hydrogen peaks for all the PHA Heel Flowsheet development runs. The "Small PHA Heel" runs are the first six experiments using the existing late wash flowsheet. The "Large PHA Heel" runs are the six original large PHA heel runs in the 4 liter stainless kettle. The "PHA Heel Tests 1-7" runs are the seven additional large PHA heel runs in the 1 liter Hastelloy™ kettle conducted to support the postulated mechanism of increasingly higher noble metal concentration in each successive PHA heel Phase II experiments. Note that the hydrogen trend for the original small PHA heel run is very similar to the hydrogen trend in the further study large PHA heel runs.
A computational study was performed to investigate the similarity between the small PHA heel process runs and the large PHA heel Further Studies runs. A worst case scenario was set up assuming that all of the noble metals contained in the PHA solution below the agitator line were left behind in the kettle. Table XII compares the predicted values obtained for the ratio of noble metals to the actual ratio of the hydrogen peak for each of the three processing scenarios. Note that the predicted ratio uses the average run 1 value of all three runs. The predicted values correspond well with the actual hydrogen ratios, which implies a strong correlation between the noble metals and the hydrogen production. This also shows that the solids settling in the kettle theory (second postulated mechanism) is most likely correct. Because the prediction shows a steady increase in noble metals concentration that reaches a steady state around the 6th run, the hydrogen level observed at the 6th run should be the highest value seen, if the settling is the only factor in the increasing levels.
Table XII. Computational Study Results
|
Predicted Ratio of Noble Metals (Run 6/Run 1) |
Actual Ratio of H2 Peak (Run 6/Average Run 1) |
|
|
Small PHA Heel Process |
1.25 |
1.23 |
|
Large PHA Heel Process |
1.63 |
1.62 |
|
Large PHA Heel Tests 1-7 |
1.23 |
1.22 |
ACKNOWLEDGEMENTS
Thanks to Frances Williams, whose many hours of technical coverage (and working with our gas chromatographs) were invaluable to the success of these experiments.
A special thanks to the ITS technicians, Sammie King, John Duvall, Mary Johnson, Vickie Williams, for their hard work in accomplishing these runs. In addition to working shifts to provide coverage throughout the runs, they set up equipment, calibrated analyzers, prepared chemicals, and performed analytical work to support this study. Thanks also to Shumei Lee, who also provided technical coverage during the runs.
Thanks to Grace Hsu and Curtis Johnson for their assistance in completing the analytical work during the runs. Thanks especially to Tom White and Annie Still for their HPLC work.
Thanks to Tommy Edwards, who provided the statistical support and completed the statistical study. Thanks also to Paul Monson, Russ Eibling and Jim Marek, who provided technical insight throughout the study.
REFERENCES