
A METHODOLOGY FOR DOSE RESPONSE EVALUATION
THAT ADDRESSES STAKEHOLDERS’ CONCERNS
Anthony E. Hechanova, Elizabeth A. Johnson, and Sun-Ho Pensinger
Harry Reid Center for Environmental Studies
University of Nevada, Las Vegas.
ABSTRACT
This paper discusses the recommendations of the Health Physics team of the Nevada Risk Assessment/Management Program (NRAMP) for refinement of dose response evaluation methods in order to respond to stakeholder concerns. The approaches are intended to provide a consistent framework to evaluate radiological risks from the variety of potential contaminants and exposure pathways at the Nevada Test Site using the latest scientific evidence. All too often, the true nature of the scientific controversy surrounding dose response in the low-dose region (defined in this paper as an annual dose less than 5 cSv) is not clearly conveyed to non-technical constituencies and technical people whose expertise is outside the realm of health physics. This paper relates the method and implementation of dose response communication between the NRAMP Health Physics team and diverse stakeholder groups, and gives guidance for estimating the range of risk prediction in the low-dose region. Since this range includes health detriment and benefit, the authors recommend quantifying results in the low-dose region using dose estimates. Such results will have a lower uncertainty and can be conveyed by comparison to background exposures of radiation, regulatory levels, and scientific standards.
INTRODUCTION
Nuclear device testing in Nevada has produced a very unique situation in which radioactive material that is typically under strict control has been intentionally released into the environment. There are very few places (possibly the testing grounds for the former Soviet Union) that can compare to this legacy at the Nevada Test Site (NTS). The NRAMP is a joint effort of the Harry Reid Center for Environmental Studies at the University of Nevada, Las Vegas, and the firm of E. J. Bentz and Associates, Inc., of Springfield, Virginia, funded by a U. S. Department of Energy (DOE) grant (No. DE-FG01-96EW56093) to conduct independent stakeholder-based risk assessments and risk management evaluations of DOE environmental management activities in Nevada. The principal product of NRAMP Phase I, the Preliminary Risk Assessment (PRA)(1) was discussed at WM97(2). The PRA was a cursory analysis to identify the nature and extent of the radiological hazard, and provided qualitative and quantitative information about current and future public health risks from activities related to nuclear weapons testing at the NTS. It used a single dose-response curve (the linear no-threshold model) and a single end-point (stochastic radiation carcinogenesis for Reference Man (3)) which allowed consistent relative risk comparison between source categories to a maximally exposed hypothetical individual. The PRA was useful in providing future NRAMP direction and in identifying where significant gaps in data exist and where large uncertainties in NRAMP risk assessment methodology may have significant impacts. However, the use of simple assumptions in the estimation of risk, without regard to uncertainty or reliability, tends to imply precision beyond actuality and to promote miscommunication and misinterpretation between scientists and stakeholders. Possibly the biggest challenge to NRAMP is accurately portraying the context of results and their concomitant uncertainty to a non-technical audience.
The authors have found that a key component to successful stakeholder communication is an honest and transparent process. Public stakeholders have been intimately involved in NRAMP since its inception in 1995 through telephone surveys, working groups, focus groups, university classes and symposia, and one-on-one communication. Unfortunately, with respect to radiation risk, the true nature of the scientific controversy surrounding dose response in the low-dose region gets lost and misrepresented when interdisciplinary groups rely on the simplicity of radiation protection standards which purport public protection as opposed to scientific integrity. These methodologies may be appropriate for regulatory compliance, but may not accurately answer the questions of decision makers and public stakeholders. It therefore behooves a program like NRAMP to consider the array of complexities involved with dose-response modeling and the evidence in support of other dose-response models to give credence to quantitative risk calculations and uncertainty analyses.
BIOLOGICAL RESPONSES AT BACKGROUND LEVELS
Evaluation of radiological risk requires quantifying the dose deposited to critical cells and tissues and relating this dose to subsequent potential health effects. High radiation levels undoubtedly incite biological responses such as chromosome aberrations, nausea, hair loss, vomiting, and a drop in blood cell count. However, scientific observation is lacking for biological responses from radiation exposures comparable to, or less than, background radiation levels. In fact, scientists propose several contradicting theories regarding low-dose responses.
It is crucial to convey to stakeholders that radioactivity is a natural phenomenon that occurs everywhere, i.e., in the air, in the ground, in food, and even in the components of the body, and that it is important to understand and consider background levels of radiation dose in order to put radiological risks in their proper context and perspective. The authors have found it useful to use regional background dose values, such as shown in Table I for the two major metropolitan areas of Nevada, to give stakeholders a visceral connection to dose values and the large variation produced by nature.
Table I. Total Natural Background Radiation Dose in Nevada

The concept of background levels is also important to convey during discussion of stochastic effects (namely, cancer and genetic mutations) in the low-dose region. Important facts to provide stakeholders are (1) cancer is a very common illness that is actually about 100 different diseases characterized by the uncontrolled growth of cells; (2) in the United States, one out of every three people will contract cancer and one out of every five people is expected to die from cancer; and, (3) there are roughly 1000 different chemical carcinogens so that radiation is not the only consideration. These facts help to convey that there is a "background" incidence or risk of a fatal cancer of one in five or 0.2 probability that is not merely attributable to radiation.
Likewise, genetic mutations are very common in humans although the mutation does not always manifest itself in a visible fashion. Exposure to background radiation produces about one billion mutations per day in DNA which is a small percentage of the about ten thousand billion mutations per day caused by normal metabolic and DNA replication processes of all the cells in the body(4). Radiation protection studies, such as by the International Commission on Radiological Protection(5), conclude that the risk value for latent fatal cancer from a radiation dose is larger (by a factor of about five) than the risk value for a severe hereditary effect from the same exposure.
LOW-DOSE MODELS
Biological responses to low doses of radiation are difficult to observe because of the many natural and human-made carcinogens and mutagens that individuals are subject to on a daily basis. In fact, the debate is raging in the scientific community as to the extent of radiologic cause and even if such effects known to occur at high doses are manifest at low-dose exposures. The basic character (e.g., shape) of the dose-response relationship is at the center of the issue. Scientists represent the dose-response relationship using simple curves based on their interpretation of evidence. The majority of dose-response theories are contained in one of the five curves shown in Fig. 1. The three major theories are the linear no-threshold (which includes the supra-linear and linear-quadratic variations also shown in Fig. 1), threshold, and hormesis assumptions. A realistic model is probably more complex than such simple models. Factors such as cell type, fractionation, dose rate, and linear energy transfer (LET) can have large effects on values of risk parameters in models.
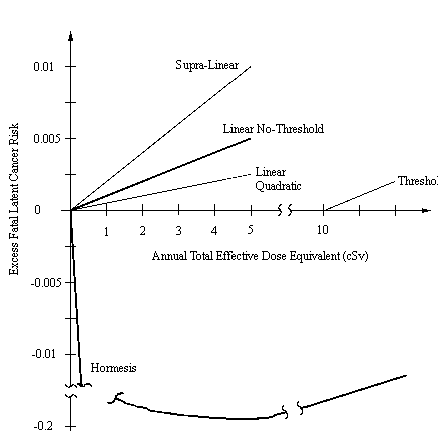
Figure 1. Examples of dose-response curves for latent cancer fatality in the low-dose region.
It should be noted from Fig. 1 that the various theories all converge in the high-dose region where scientific evidence is very clear and high-dose theories are uncontested. However, the theories contradict each other in the low-dose region and, depending on the assumptions chosen, dramatically different risk conclusions can be drawn. The goal of NRAMP is to be responsive to the variety of stakeholders, from the most pessimistic to the most optimistic. Therefore, the following theories are presented individually and neutrally by the NRAMP Health Physics team to stakeholder groups.
Linear No-Threshold
The linear no-threshold model, as its name implies, assumes that every radiation interaction will have associated with it an increased probability of producing a deleterious effect; and, the excess probability is linearly proportional to the amount of dose absorbed. According to the Commission on Biological Effects of Ionizing Radiations (BEIR)(6), all forms of radiation cause a response in cells as evident by chromosome aberrations induced by relatively low doses of radiation. The risk values for this model are based primarily on data from the Japanese atomic bomb survivors. According to Hill et al.(7), after an exposure to low levels of radiation, there is no increase in cell killing and only a small induction of neoplastic transformation. This suggests that at very low dose rates the cell’s repair mechanisms effectively remove or nullify most of radiation damage. However, Watanabe et al.(8) conclude that although chronic irradiation might increase the growth potential of cells, this is not necessarily a positive adaptive response as the same cells have a cumulative burden of chromosome changes. Those who support the linear no-threshold model believe that it is apparent from the various experiments that no threshold for radiation effect exists. Cells have efficient repair mechanisms, but survival may be at the cost of future genetic effects and/or cancer.
Scientific organizations which ascribe to the linear no-threshold model for radiation protection, such as the ICRP(5), BEIR(6), the National Council on Radiation Protection and Measurements (NCRP)(9), and the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR)(10), state that low doses and low LET radiation are not as effective in cell killing as high doses. Therefore, they apply a Dose and Dose Rate Effectiveness Factor (DDREF) as a "reduction factor" to extrapolate high-dose data to the low-dose region. DDREF ranges from these reports are between 1 and 10. In determining the choice of the DDREF value for low LET radiation, the ICRP(5) notes: (1) that the full range of DDREF values (from 2 to 10) obtained from animal studies may extend over a broader dose range than human data and therefore include higher values than are relevant; (2) that some human experimental data shows little evidence of fractionation effects while others indicate possible factors of up to 3 or 4; (3) that direct statistical assessment of the Japanese atomic bomb survivor data does not seem to allow for much more than a factor of about 2 for the DDREF; and, (4) the DDREF ratios recommended in the past from other organizations suggest that, in lieu of the limited human information available for the low-dose region, a DDREF of 2 is appropriate for purposes of radiation protection. ICRP(5) recommends this factor for low-LET absorbed doses below 0.2 cSv and for higher absorbed doses when the dose rate is less than 0.1 cSv per hour.
Linear Quadratic
The linear quadratic, no-threshold model has been used to describe many radiation biology experimental results. This model is written mathematically as:
S - S0 = a D + b D2
where (S - S0) is the observed excess response above S0, the spontaneous response. D is the absorbed dose and a and b are coefficients that depend on many factors such as cell type, dose rate, and LET(6). According to Edwards(11), the results of the leukemia studies of the Japanese atomic bomb survivors show that the linear-quadratic model provided the best fit of the data. By applying the reduction factor (DDREF) to the linear no-threshold extrapolation from high-dose data, the predicted response resembles the linear quadratic curve in the region around background levels (i.e., on the order of 0.5 cSv where the quadratic term is much smaller than the linear term). However, it is important to recognize that this equation only applies to the low-dose region below about 10 cSv.
Supra-Linear
The supra-linear theory, advocated by Gofman(12), asserts that low doses of radiation produces effects above and beyond a linear-extrapolated response from high-dose data, contrary to the linear-quadratic model. In other words, the supra-linear assumption proposes that dose received from low dose rates of radiation are more hazardous than acute high doses for the same amount of accumulated dose. Gofman performed an independent investigation of the Radiation Effects of Research Foundation’s (RERF) data on the Japanese atomic bomb survivors. He found that the supra-linear model provided the best fit of the data and points to the RERF analysts’ findings that the linear and supra-linear curves fit the data (excluding leukemia) equally well. Gofman asserts that ionizing radiation has proven to cause double DNA strand breaks and induce carcinogenesis so that low doses of radiation can cause genetic damage which is unrepaired, unrepairable, or misrepaired. In addition, radiation-induced chromosome damage does not always kill the cell which may go on to divide and replicate the abnormality. The subsequent cell lines may cause the formation of malignant cells. The reason prolonged exposure to even low doses of radiation is hazardous is that residual damage exists which can result in cancer and protracted exposure to low doses cause more damage than acute high-dose exposures. Gofman suggests that the high background incidence of human cancer could be partially explained by chronic background radiation. He therefore recommends using a risk enhancement factor of 2 on the linear no-threshold coefficient extrapolated from high-dose data to essentially model the supra-linear assumption.
Threshold
Several scientists and organizations believe that no harmful effects are produced below a threshold dose of radiation because of the body’s ability to repair or defend against sub-lethal damage caused by low doses of radiation. The Health Physics Society Position Statement(13) recommends using a practical threshold for radiation protection purposes since no radiation effects are observed below a dose of 10 cSv. The threshold model assumes that there is no response up to some dose level, and that, thereafter, the response is proportional to dose. The adaptive responses of cells are effective and sufficient to repair damage below 10 cSv such that no deleterious effects will occur. In fact, according to Meyers et al.(14), some proteins are induced specifically by radiation, and not by other stress agents. It is these proteins that are presumed to be involved in repair or regulatory functions and whose induction may prevent or remove some of the damage from subsequent larger doses of radiation. Olivieri et al.(15) state that small priming doses of radiation can induce an adaptive response in human lymphocytes that reduce the amount of damage from exposure to larger doses. With all the defensive processes available to the cell and organism, the damage caused by low doses of radiation should be easily repaired or moderated. Thus, below a certain threshold dose, no effect is observed as the damage present is repaired with no deleterious effects to the individual.
Hormesis
The hormesis theory endorsed by Luckey(16) is an extension of the threshold model and states that background radiation is necessary and beneficial for human health. Therefore, the hormesis model assumes that zero dose is actually harmful because the body needs low doses of ionizing radiation to activate its defense mechanisms, including increases in cell proliferation, enhanced DNA repair, and up-regulation of the immune system. The end result of this stimulation process is increased lifespan and reduced cancer mortality. In the low-dose region (around 1 cSv or less), Luckey predicts a nominal coefficient of 5 x 10-2 fewer cancer deaths per cSv which eventually bottoms out at about 5 cSv as shown in Fig. 1. Other studies supporting hormesis include a ten-year study by the Johns Hopkins Department of Epidemiology on nuclear shipyard workers that indicated they had lower death rates from all causes (e.g., leukemia, lymphatic, and hematopoietic cancers) than non-nuclear workers(17), and epidemiological studies conducted by the U.S., Sweden, and China, which show regions with high radon concentrations compared with averaged background rates have lower incidences of cancer(18, 19, 20, 21). These results contradict the risk predicted by the linear no-threshold model.
DISCUSSION AND RECOMMENDATIONS
Risk evaluations from radiologic dose needs to be divided into at least two regions: high-dose (above approximately 10 cSv) and low-dose (below approximately 1 cSv). Any level chosen for this demarcation will find detractors, therefore, the authors of this paper recommend (somewhat arbitrarily) using an annual dose of 5 cSv as a demarcation to define between low-dose and high-dose regions. This choice is based on the following considerations: (1) being a middle-value clearly above background levels and below non-stochastic thresholds, (2) being representative of the federal limit on allowable dose to individuals from occupational exposures, and (3) consideration of statistically meaningful calculations. According to Adams(22), considering both systematic modeling errors and uncertainties in dosimetry data (and other factors leading to the determination of risk coefficients), the minimum calculable significant risk exceeds 0.01 for the usual confidence limits. He states that with careful error analysis, lower uncertainty levels may be achieved but it is unlikely that they will lie below an excess risk value of 0.001. These statistically meaningful risk values correspond to doses between 1 and 10 cSv. In other words, radiological risk assessments will not be able to estimate risks from doses less than about 5 cSv with scientific confidence because uncertainties will be larger than any calculated values of risk. The limitation on scientific risk assessments resulting from uncertainties in the understanding of radiological dose and risk is an important concept because it highlights that (1) the low-dose response controversy is currently not resolvable because it falls below a region of scientific certainty and (2) the controversy itself is justifiable because the differing theories cannot be refuted with statistically significant observations.
High-Dose Region
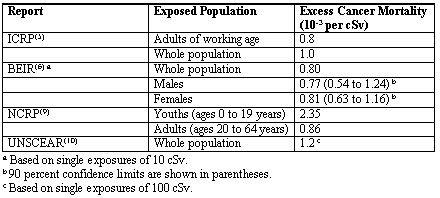
Above 5 cSv per year, the authors recommend evaluating fatal cancer risk using a coefficient that is consistent with the radiation protection organizations’ analyses of the high-dose data for all cancers in whole populations: 1 x 10-3 per cSv (see Table II). A 95 percent confidence range of ± 50 percent is recommended. If a risk assessment is to consider the effects of gender and age in dose response, the BEIR report(6) indicates that there is only a slight difference between male and female risk coefficients which does not appear to be significant. However, NCRP(9) indicates that probabilities for fatal cancers vary with age, and the total risk is greater by a factor of about three in the 0 to 19-year age group than the 20- to 64-year age group. Although this factor may not make a significant difference in total lifetime risk, it could be important in answering stakeholder concerns regarding children.
Table II. Risk Coefficients Reported by Various Radiation Protection Committees
Low-Dose Region
Scientific evidence is inconclusive in the low-dose region (below 5 cSv per year). In order to be responsive to various stakeholder opinions, from those who ascribe to the most optimistic hormesis theory to the most pessimistic supra-linear theory, the authors feel it is important for the risk assessor to indicate to their audience that the uncertainty is greater than any values for risk and that plausible theories have a wide range. This range varies at the lower end of the low-dose region below 1 cSv from a nominal coefficient of 5 x 10-2 fewer cancer deaths per cSv (hormesis assumption) to 2 x 10-6 excess cancer deaths (supralinear assumption). A range between positive and negative risk (i.e., health benefit or health detriment) may be unsatisfying to stakeholders; therefore, the authors feel that quantitative results should be dosed-based since the controversy and inconsistency in theories stem from the evaluation of dose response, not from the more uniformly-accepted calculation of dose. The agency or stakeholder group can then determine their risk assessment purpose, e.g., public health protection, prediction, or priority setting. For example, if the assessment is performed for regulatory compliance, then radiation protection values will most likely be stipulated. If the assessment is performed to answer public stakeholder concerns, then risk evaluation is highly uncertain because scientific evidence is inconclusive and dose-response theories in the low-dose region are contradictory. Thus, the authors recommend reporting dose-based results which are uniformly accepted in the scientific community and can be accurately conveyed to a non-technical audience (e.g., by comparison to background exposures of radiation).
CONCLUSIONS
A key to successful stakeholder communication is an honest and transparent process. This applies to the dose response evaluation component of radiological risk assessments and this paper has outlined major concepts used by the authors in their interactions with Nevada stakeholders. A demarcation of 5 cSv was selected to define between the low-dose and high-dose regions based on the following considerations: (1) being a middle-value clearly above background levels and below non-stochastic thresholds, (2) being representative of the federal limit on allowable dose to individuals from occupational exposures, and (3) consideration of statistically meaningful calculations. In the high dose region, the authors recommend evaluating fatal cancer risk using methods that are consistent with radiation protection organizations’ analyses of the high-dose data for cancer. In the low-dose region, an accurate portrayal of the scientific controversy should be given including theories for health detriment and benefit. Since a positive and negative risk value may be unsatisfying to stakeholders, the authors recommend quantifying results in the low-dose region using dose estimates. Such results will have a lower uncertainty and can be conveyed by comparison to background exposures of radiation, regulatory levels, and scientific standards.
REFERENCES