IMMOBILIZATION OF ASH RESIDUE FROM RADIOACTIVE
WASTE INCINERATION INTO GLASS - LIKE MATRIX
Galina A. Varlakova, Olga K. Karlina, Viktor M. Tivansky,
Michael I. Ojovan and Sergey A. Dmitriev
Scientific and Industrial Association "Radon"
The 7-th Rostovsky Lane
2/14, Moscow, 119121, Russia
Tel. (095)248 1680
Fax (095)248 1941
E-mail oj@nporadon.msk.ru
ABSTRACT
In this work there was studied a possibility of solidification of ash residue from solid radioactive waste incineration in the process of solid - phase chemical reactions between components of thermal base (heating batch) and ash residue.
INTRODUCTION
The problem of conditioning of ash residue from solid radioactive waste incineration is highly urgent. It is solved by various methods including vitrification. Immobilization of ash residue into glass and glass - like materials is connected, as a rule, with its melting with glass - forming additives or ash residue melting, for which purpose sufficiently complicated melting equipment and significant energy consumption are required [1,2].
Method of solidification of ash residue from solid radioactive waste incineration proposed in this report is realized in a crucible-container without energy supply from external heat source to the system. Heat liberated in the process of solid-phase chemical reactions between components of heating batch and ash residue goes to melt ash residue and to form monolithic glass-like material.
Heating batch is a mixture of reagents liberating heat during redox exothermic reaction in amounts required for ash residue melting without energy supply from external source. The empirical formula of heating batch is Ca1,9280 Fe1,3836 Si10,6815 K2,8763 Al3,3693 Mn2,8763 O11,5050 . The heating batch of mentioned composition liberates, in certain relation of the components, heat on the order of 5 MJ/kg and provides reacting mixture temperature on the order of 1600 - 1800 oC.
EXPERIMENT
In the experiments the following composition of simulated ash residue was used , wt.% : 6 Na2O, 9 K2O, 14 CaO*, 5 MgO, 15 Al2O3, 10 FeOx, 30 SiO2, 11 P2O5* ( *Oxides were introduced in the form Ca3(PO4)2). Actual ash from radioactive waste incineration facility was used in the experiments as well. Specific activity of ash residue was S b 137 Cs = 3,4*105 Bq/kg, S a 239 Pu= 1,6*105 Bq/kg.
The heating batch and ash residue were used in the experiments in the ratio 40-50 : 60-50.
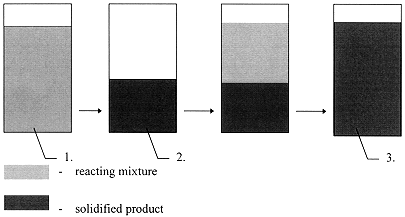
The experiments were carried out under laboratory conditions in alundum crucibles 1 dm3 in volume. The mixture mass ranged from 0,5 to 1,0 kg. The heating batch was carefully mixed with ash residue, resulting mixture was placed in the crucible and initiated by fuse or electric igniter. Mixture combustion wave was propagated downward and at the same time liquid melt was formed. Then liquid glass - like material was cooled under normal conditions and on cooling it became dense glass - like monolith. By proceeding to do this over and over again the container was completely-filled with glass-like material (Fig. 1).

Figure 1. Scheme of Filling of the Crucible-Container: 1 - container with reacting mixture, 2 - container with solidified product, 3 - completely-filled container
The process temperature was registered by the use of Chromel - Alumel thermocouple placed in the initial mixture mass. The typical curve of time - dependence of the process temperature is shown in Fig. 2. The residence time of material at 1000 0C and higher ranges from 10 to 40 minutes for different compositions and reacting mixture mass.

Figure 2. Temperature of the Process (oC) vs Time.
With the aim of estimating macrocomponents and radionuclides carry - over the experiments were carried out in the special exhaust facility (Fig. 3) which provides the sampling of the overall aerosol phase generated from the process.
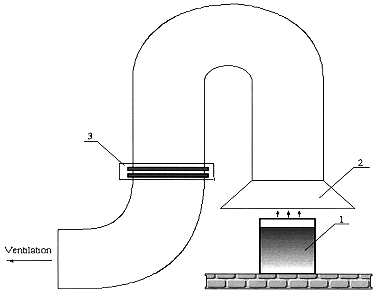
Figure 3. Scheme of the Exhaust Facility: 1 - crucible-container with reacting mixture, 2 - hood-gas collector, 3 - cassette with aerosol filters.
Photomicrography of the glass-like material samples containing 50, 56 and 60 wt.% of ash residue was performed using HITACHI scanning microscope S-4OSA of 300-3000 power.
X-ray diffraction analysis of mentioned samples was carried out by the use of diffractometer DRON - 1,5 (Cu Ka - emission).
Water durability of the samples was estimated from the leaching rates of sodium and cesium-137 under prolonged contact with distilled water determined by the procedure presented in [3 ].
RESULTS AND DISCUSSION
Temperature being increased in the course of the reaction of heating batch and ash residue depends on ash residue content in the mixture. It has been noted that maximum reaction temperature for the mixture containing 50 wt.% of ash residue decreases from 1400 0C to 1200 0C for the mixture containing 60 wt.% of ash residue. In all cases the process produces monolithic glass-like black-colored material.
Aerosol carry-over in the course of the process was determined using a facility (Fig. 3). Sampling of aerosols was made by Petryanov filters of type AFA-VP. Aerosol carry-over ranged from 0,75 to 3 %. Amount of aerosols depends on temperature and mixture composition and decreases with increasing ash residue content in the mixture and with decreasing process temperature.
X-ray diffraction analysis of final product samples shows that high-temperature interaction of ash residue and components of the heating batch occurs which results in formation of amorphous phase. The samples have, among amorphous phase, crystalline inclusions representing kaliophilite (KAlSiO4). From the intensity of peaks and size of diffuse halo on X-ray photographs it is obvious that portion of amorphous phase in the final product increases with decreasing ash residue content and, respectively, amount of crystalline inclusions decreases.
From the electron-microscopic photographs of ash-containing final product samples the glass formation area with microcrystalline inclusions can be seen. Amount of these inclusions increases with increasing product filling with ash residue (Fig. 4).
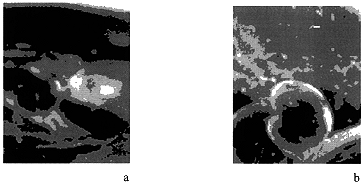
Figure 4 . Electron-microscopic Photographs (300x magnification) of the Glass-like Materials with Ash Residue Content: 50 wt.% (a) and 60 wt.% (b).
Leaching rates of Na+ and 137Cs- ions from samples of different filling with ash residue at 21st day of testing are given in the Table I. Water durability of mentioned samples is higher than one of glass-like materials produced by analogous process with the use of heating batch of another composition about which it has been reported in[4].
Table I. Results of Testing of the Final Product for Water Durability.
Mixture composition, wt.% |
Leaching rate at 21st day, g/cm2*day |
||
Ash residue |
Heating batch |
137 Cs |
Na+ |
60 |
40 |
7,0*10-5 |
7,9*10-5 |
56 |
44 |
2,8*10-5 |
4,9*10-6 |
50 |
50 |
5,4*10-6 |
9,0*10-6 |
CONCLUSIONS
By this means the results of the experiments indicate the principle possibility of immobilization of up to 60 wt.% of ash residue into glass - like matrix without using external sources for system heating.
Properties of the final product of ash residue solidification and radionuclide carry-over in the process are determined by the degree of matrix filling with ash residue.
Properties of the final product may be improved through optimization of composition of the heating batch; introduction of glass-forming components, fluxing additives into it.
REFERENCES