ELECTROCHEMICAL DECONTAMINATION SYSTEM FOR
ACTINIDE PROCESSING GLOVEBOXES
D. E. Wedman, J. L. Lugo, D. Ford, T. O. Nelson, V. L. Trujillo, H. E.
Martinez
P.O. Box 1663, MS E510, Group NMT-6
Los Alamos National Laboratory
Los Alamos, NM 87545
ABSTRACT
An electrolytic decontamination technology has been developed and successfully demonstrated at Los Alamos National Laboratory (LANL) for the decontamination of actinide processing gloveboxes. The technique decontaminates the interior surfaces of stainless steel gloveboxes utilizing a process similar to electropolishing. The decontamination device is compact and transportable allowing it to be placed entirely within the glovebox line. In this way, decontamination does not require the operator to wear any additional personal protective equipment and there is no need for additional air handling or containment systems. Decontamination prior to glovebox decommissioning reduces the potential for worker exposure and environmental releases during the decommissioning, transport, and size reduction procedures which follow.
The goal of this effort is to reduce contamination levels of alpha emitting nuclides for a resultant reduction in waste level category from High Level Transuranic (TRU) to Low Specific Activity (LSA, £ 100 nCi/g). This reduction in category results in a 95% reduction in disposal and disposition costs for the decontaminated gloveboxes. The resulting contamination levels following decontamination by this method are generally five orders of magnitude below the LSA specification. Additionally, the sodium sulfate based electrolyte utilized in the process is fully recyclable which results in the minimum of secondary waste.
The process has been implemented on seven gloveboxes within LANL's Plutonium Facility at Technical Area 55. Of these gloveboxes, two have been discarded as low level waste items and the remaining five have been resused.
BACKGROUND
The removal of actinide processing gloveboxes from service occurs due to changing missions, facility closings, and facility upgrades. Programmatic requirements at LANL require the decommissioning of obsolete gloveboxes contaminated internally with high levels of transuranic (TRU) radioisotopes. At least 300 gloveboxes at LANL are expected to be decommissioned over the next 5 years and more over the long term. In addition to these active gloveboxes that are in need of decommissioning, there are also on the order of 200 "legacy" TRU categorized gloveboxes in temporary storage.
Other DOE sites also need to decommission gloveboxes, for example, Rocky Flats Environmental Technology Site (RFETS) has in the range of 1000 gloveboxes requiring decommissioning. The Idaho National Engineering Laboratory plutonium line and Oak Ridge National Laboratory uranium line also require some amount of decommissioning. The construction material of these gloveboxes is, nearly without exception, stainless steel – most commonly type 316 stainless steel.
In general, the process of decommissioning a TRU categorized glovebox involves disconnection from the facility, packaging, transportation to a size reduction facility, unpacking, size reduction, packaging for disposition, and, ultimately, disposal at a long term storage site like the Waste Isolation Pilot Plant (WIPP). For LANL, the estimated cost subsequent of disconnection is about $140,000 per average sized TRU glovebox.
Disposal cost is primarily related to the level of contamination contained within the glovebox. By decontaminating to LSA levels (£ 100 nCi/g), glovebox disposal cost is reduced to approximately $6,500 per glovebox, a 95% savings. Reduction in waste category has several other benefits. LSA categorized gloveboxes have an immediate path forward for disposition – they may be disposed of in approved landfill sites. For TRU gloveboxes, because WIPP is not yet open, the boxes must be stored in temporary storage for an unknown period of time.
Additionally, decontamination enables the reuse of gloveboxes that are not considered obsolete by design. Gloveboxes with lower than TRU levels of contamination may be stored temporarily within the LANL Plutonium Facility and are therefore candidates for reuse. Occasionally, these gloveboxes may even be reused without facility disconnection and subsequent reconnection. This is true if decontamination was only necessary to lower worker radiation exposure related to glovebox holdup, or because new processing operations within the glovebox would be effected by the existing level of contamination.
There are other benefits for carrying out the decontamination prior to disconnection from the facility in terms of a reduced risk to workers, the public, the facility, and the environment if decontamination occurs before the glovebox is disconnected from the facility air handling system.
INTRODUCTION
A method LANL has adopted and developed to accomplish the task of lowering TRU categorized gloveboxes to LSA category gloveboxes is an electrolytic method. Through this process, atomic surface layers of the metal are electrochemically removed from the material by a mechanism very similar to the wide spread industrial technique of electropolishing.
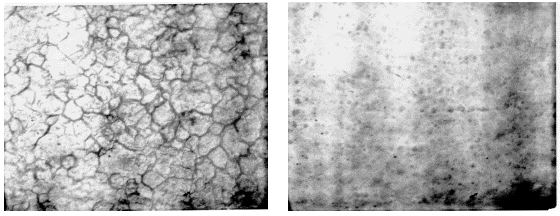
Electropolishing is a common metallic surface preparation technology that produces a microscopically smooth surface without abrasives. The resulting surface has many desirable characteristics including enhanced corrosion resistance and a high luster which is shown in Figure 1.

Figure 1. Typical cold-rolled stainless steel surface at approximately 500X magnification (left) and same surface following electropolishing (right), same magnification.
Industrial scale electropolishing processes are carried out using large heated vats of electrolyte solutions into which the item to be polished is immersed. Once immersed, a current (nominally ³ 100mA/cm2) is applied between the object being polished and other electrodes immersed in the same tank. The object being polished is biased to positive voltages with respect to the other electrodes. This causes the surface layers of the metal to be anodically dissolved. The electrolytes commonly used for such processes are tailored to the metal being polished and in general consist of highly acidic solutions at high (³ 100° C) temperatures. For stainless steel, the electrolytes normally are composed of concentrated solutions of phosphoric or sulfuric acids mixed with other acids and/or glycerin.
Early research in applications of electropolishing to surface decontamination by Allen et al. (1) at Pacific Northwest Laboratory focused on the use of these commercial electropolishing solutions for decontaminating a variety of metallic surfaces. For applications in the nuclear industry, however, the use of standard industrial electropolishing baths generates unacceptably large volumes of highly acidic radioactive waste that must eventually be treated in some manner and discarded.
Later, Childs and Winkel (2) demonstrated that alkaline solutions containing high concentrations of sodium nitrate as the electrolyte could be utilized to decontaminate stainless steel surfaces. The advantage to these solutions was in the area of waste minimization. Under the alkaline solution conditions, the metals and actinides form a precipitate which are removed from the electrolyte and the electrolyte is recycled.
The work by Childs and Winkel was successfully adapted for use in decontamination of highly enriched uranium (HEU) weapons components in an engineered flowing system, but the electrolyte was found to be inadequate for decontamination of stainless steel due to preferential oxidation of welds, stressed, or otherwise energetic areas, an innate proneness to surface pitting, and the formation of various nitrogen species due to oxidation and reduction of nitrate which limits electrolyte lifetime.
Other potential electrolytes were examined until it was found that electrolysis in an alkaline solution of a sulfate salt (e.g., sodium sulfate) resulted in good surface characteristics (no preferential attack at welds, stressed, or otherwise energetic areas, showed no signs of pitting) and was electrochemically inert under the conditions of the electrolysis. This discovery has allowed the application of this technology to steel surfaces, e.g., gloveboxes, utilizing an engineered system which minimizes solution volume and requires only small dc voltages and currents.
Although it is not the intent of this paper to compare decontamination technologies, it must be pointed out that other decontamination techniques have been investigated for decontamination of gloveboxes and other materials. Principle among these are decontamination techniques based on blasting (abrasives, CO2 pellets, and high pressure water), chemical etches (acid washes with or without chelating agents), and plasmas.
With the exception of plasma technologies and CO2 blasting, these approaches in general produce large quantities of waste which requires treatment and/or disposal. CO2 blasting and high pressure water sprays do not lend themselves to in situ decontamination of gloveboxes because CO2 gas can overwhelm negativity of glovebox system and high pressure water can shred glovebox gloves. Plasma technologies are likely to be many years away from implementation in actinide contaminated gloveboxes.
CHEMISTRY
Being comprised primarily of iron, nickel, and chromium, the anodic dissolution of stainless steel results in the generation of cationic forms of these elements within the electrolyte solution. As the surface layers of the metal are dissolved, the contaminants within those layers are freed from the surface, a phenomenon that results in decontamination of the surface.
In order to recycle the electrolyte solution, the radioactive components freed from the surface as well as the cations generated in the process must be removed. Because both iron and nickel are insoluble in alkaline solutions – precipitating as hydroxides under these conditions – the sodium sulfate electrolyte is maintained at a high pH through the addition of sodium hydroxide. The formation of these precipitates is key to the process. Not only does the precipitation result in the removal of these elements from solution, but the precipitate additionally entrains or "captures" the actinide particles that have been released from the metal surface. A mechanical filtration of the electrolyte solution results in a decontamination of the electrolyte.
There is a debate on the mechanism of decontamination. Most contamination in the glovebox environment is as particulate oxides of plutonium. These species may have some solubility in acidic solutions. One may argue that although the bulk solution pH is alkaline, the pH at the surface may be quite low due to water oxidation as discussed later. It is therefore possible that the contamination is chemically dissolved and precipitates in solution as it diffuses from the surface. The other possibility is that particulate contamination is mechanically freed from the surface as the underlying metal is anodically dissolved, thereby remaining in particulate form. The actual pathway is unknown at present, but is likely to be some combination of the two mechanisms. Further studies are planned to ascertain this mechanism.
Unlike iron and nickel, the hexavalent chromium ion does not precipitate. Instead, it reacts with hydroxide ions to form a soluble chromate (CrO42-) species. Chromate is typically removed from solution by a secondary process in which the hexavalent chromium is chemically reduced to a trivalent oxidation state which in turn precipitates as chromium hydroxide (Cr(OH)3).
Because chromium is more closely regulated than the other metals that make up stainless steel, its removal from the precipitate subsequent to the removal of the other metals and radioactive contaminants is desired. It is possible to precipitate chromium in situ during the decontamination process by reduction of the chromate to trivalent chromium by in-line addition of a reducing agent. The advantage to this methodology is that the chromate concentration in solution is always kept small and therefore the precipitate requires little rinsing to remove the hazardous component. (Trivalent chromium is less hazardous and therefore less regulated than hexavalent chromium.) This decontamination system currently does not employ in situ reduction of chromate, instead, chromate is precipitated in a batch process. Chromate is not found to interfere with the electrochemistry process up to at least the g/l concentration, though it is not yet known if the chromate has an effect on the decontamination efficiency. This data is still being collected.
Besides the separation of the metals and contaminants from the electrolyte solution, there is another advantage to using alkaline sulfate solutions in the decontamination process. Lower voltages and currents are required than in conventional electropolishing to obtain a very uniform etch with some polishing characteristics. The voltages and currents applied to the cell are small, on the order of 3 to 10 volts and 40 to 100 milliamperes per square centimeter of surface area. This compares to electropolishing that is typically carried out at an order of magnitude greater currents and voltages. Figure 2 illustrates the difference observed in a stainless steel surface after electrolysis for a prolonged time in the alkaline sodium sulfate electrolyte.
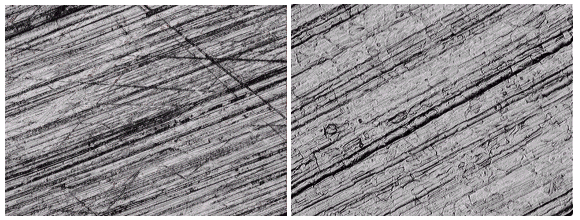
Figure 2. The surface on the left is a deep drawn type 304L stainless steel prior to electrolysis in alkaline sodium sulfate. The surface on the right is the same surface following electrolysis. Approximately 20 mg of material per square cm of surface were removed. (Magnification approximately 200X).
In the decontamination process, hydrogen and oxygen gases are generated through the reduction and oxidation of water respectively. The quantities of these gasses generated are small and deemed inconsequential for the operating currents and the standard airflow conditions within the glovebox. It is important to note, however, that up to 85% of the current at the anode surface contributes to the oxidation of water and on the order of only 15% relates to the dissolution of metal. This ratio is dependent on operating temperature, solution pH, and current density. In general, the highest metal dissolution rates occur at current densities from 40 to 100 mA/cm2, temperatures below 50° C, and low pH. Current efficiency for the metal dissolution process is found to be relatively pH insensitive for the pH range of 9 to 11. At elevated temperatures (³ 50° C) and lower current densities (£ 25 mA/cm2), the formation of a passivating oxide layer is observed. The decontamination system typically is operated at 40 to 100 mA/cm2, temperatures from 25° C to 50° C and a pH of 10.
THE ENGINEERED SYSTEM
The application of this chemistry to the task of decontaminating gloveboxes has required a great deal of ingenuity. In industrial electropolishing plants, the item being polished is fully submersed within a bath of the electrolyte solution. Because this is not a viable option for the decontamination of gloveboxes, an alternate approach was devised in which only a small section of the glovebox surface is exposed to electrolyte at any given time. To reap the greatest benefit, decontamination should be carried out prior to disconnection from the facility air handling system, therefore, the decontamination system has been designed to be compact and portable for placement entirely within the glovebox line. This configuration requires no additional air handling or containment systems because the existing glovebox system remains in tact throughout the operation.
All components of the system except for the power supply are placed within the glovebox line. The photo in Figure 3 is a photograph an actual prototype decontamination unit that is now operational within the LANL Plutonium Facility at Technical Area 55.
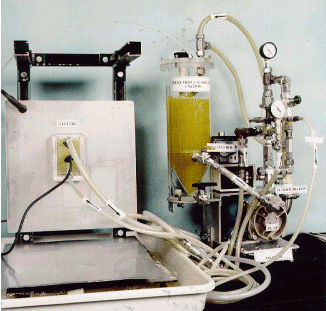
Figure 3. A photograph of a glovebox decontamination system.
The various components of the decontamination system have been refined to be as simple and robust as possible. Everything is an "off the shelf" item except for the stand, the electrolyte reservoir, the pH control electronics enclosure, the remote fixture heads, and a few various other parts.
The remote fixture heads serve as the containment reservoir for the decontamination process. This portion of the device is best described as being analogous to a steam cleaner attachment such as those used to clean upholstered furniture. These heads are where the electrochemistry takes place. There are various heads of different geometric design for decontaminating different portions of the glovebox (e.g., flat surfaces, corners, steps, protrusions, etc.).
The remote fixture heads are composed of a body of plastic or other moldable or machine workable electrically insulating material, a flexible seal, an electrode suitable for use as a cathode (e.g., a stainless steel screen) and appropriate plumbing and electrical connections. For ease in switching between different fixtures, quick disconnect fittings are utilized for the connections to the plumbing and power supply lines.
Electrolyte flow is contained within the small annulus between the fixture and the glovebox surface. The fixture is designed for uniform flow across the entire fixture surface. This volume is maintained under vacuum conditions. The source of the vacuum that maintains this condition is the high flow of solution through the eductor in the main flow loop. Typical vacuum levels are on the order of 20 inches of mercury. This vacuum serves both to seal the fixture to the surface and to pull the solutions through the fixture.
Ultrafiltration modules (A/G Technologies) are added to the system to provide a precipitate free solution to the remote fixture heads. By removing the precipitate, the radioactivity of the solution is decreased, thus providing a solution that will not recontaminate the surface being cleansed. The solution returning from the fixture head passes through the eductor and returns to the solution reservoir. The precipitate then builds up within this reservoir from which it is routinely (once or twice a day) decanted and filtered. The filtered solution is then returned to the electrolyte reservoir. To date, the same sodium sulfate electrolyte has been utilized without discard with the only notable change being an increase in concentration of the sulfate due to evaporation and the use of ferrous sulfate for reduction of the chromate.
DECONTAMINATION PROCESS
To carry out the decontamination, one or more decontamination units are brought within the glovebox line to the vicinity of the glovebox to be decontaminated. It is most desirable if the units can be placed in adjacent gloveboxes, but may be placed within the glovebox to be decontaminated without difficulty. There are no special wipe-downs or other preparation required, although it is prudent to sweep the floor of the glovebox prior to decontamination to reduce the burden on the system chemistry and to avoid introducing material to the system that may potentially foul quick connects (there is a filter in the flow loop to avoid fouling of the ultrafiltration modules).
Decontamination begins by placing the fixture head onto the contaminated surface. The fixture head immediately suctions down onto the surface, the space between the fixture and glovebox quickly filling with solution. The fixture is left in one location for a period of time. A nominal exposure time is on the order of one minute for a 100 cm2 surface area and is dependent on the level of contamination, the roughness of the surface, the nature of the contamination, and the operating current density.
Following decontamination, the fixture's vacuum seal is broken by momentarily depressing a trigger valve that lets air into the solution return line. The fixture, now freed from the surface is moved to an adjacent location. Some solution is spilled to the glovebox floor in this operation, but is readily vacuumed up for return to the reservoir by a separate return line. For this reason, the ceiling is typically decontaminated first, followed by the walls and ending with the floor. It is important to note that solution spillage does not relate to spread of contamination throughout the glovebox. The solution fed to the decontamination head is free of precipitate and therefore has very little if any contamination.
In order to limit the amount of salt residue left on the glovebox surfaces, once an area is decontaminated, it is rinsed down with a mist of water. This rinsing process serves two functions. Firstly, it rinses any salts away to where they may be recollected for continuous recycle within the electrolyte. Secondly, it replenishes water lost through electrolysis and evaporation. On average, for a single decontamination unit in operation within a glovebox, on the order of two to three liters of water are lost per day through electrolysis and evaporation.
Figure 4 depicts the decontamination process. Regions that have been decontaminated are readily identified by the renewed and brightened appearance of the metal.
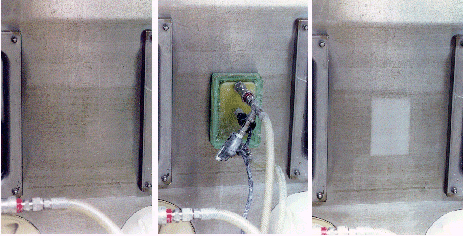
Figure 4. A glovebox surface (left) prior to decontamination, (center) during decontamination, and (right) following decontamination for one minute.
RESULTS
The decontamination system described here is just beginning its implementation lifetime. At present, two complete prototype systems are in use within the LANL Plutonium Facility. In all, seven actinide processing gloveboxes have been decontaminated and five of these have been reused within the facility with or without disconnection and subsequent reconnection to the facility systems in new locations. The remaining two gloveboxes were obsolete and therefore discarded. One of these was discarded as a Low Level Waste (LLW) item, and the other is awaiting final assay results though preliminary results also indicate LLW categorization.
It has been determined that reaching LSA is not a difficult task by this technology, but that the real technological challenge is to reach much lower levels of activity. Research is currently underway to determine how low a contamination level can be reached while a glovebox is still connected to the contaminated facility air handling system.
It has been discovered that recontamination of decontaminated surfaces due to the transport of airborne particulates by the facility glovebox air handling system is an issue for achieving very low levels of activity. In order to minimize recontamination, a replacement door has been designed for the gloveboxes within the Plutonium Facility that alleviates this problem.
Through meticulous handling techniques, experienced glovebox workers are, to date, able to bring the loose contamination levels to hundreds of decintegrations per minute per 100 cm2 and total surface contamination levels to thousands of dpm/100 cm2. These levels of contamination relate to 0.001 nCi/g of glovebox (7 gauge steel) and are all obtained while the glovebox is still connected to the facility air handling and containment systems. (This level of activity is five orders of magnitude below the LLW upper limit.)
OTHER USES
The technology utilized in glovebox decontamination has widespread applications. The same technology has been utilized to decontaminate the Special Nuclear Material (SNM) storage canisters used in both the Automated Retirement and Integrated Extraction System (ARIES) and the Defense Board's Materials Stabilization and Repackaging Project. In both of these system, stainless steel canisters loaded with Special Nuclear Materials are decontaminated externally for removal from the glovebox line, and therefore must be decontaminated to levels of surface contamination that are equivalent to free release levels (£ 500 dpm/100 cm2 direct surface measurement, £ 20 dpm/100 cm2 swipable). In these systems, the canister is placed within a specially designed fixture built into a wall separating a clean section of a glovebox from a contaminated section.
Another successful application of the technology has been the decontamination of Highly Enriched Uranium (HEU) parts from the disassembly of nuclear weapons. This process has become a DOE complex-wide standard for the decontamination of these parts and is carried out routinely at LANL. An HEU decontamination unit built at LANL is now operational at RFETS for the decontamination of their existing contaminated inventory of these parts.
Research directed at the application of this technology to the decontamination of piping and ductwork, various metallic pieces and parts, as well as other material of significance to the weapons complex (i.e., weapon disassembly) is ongoing.
REFERENCES