A NOVEL APPROACH FOR PRELIMINARY CONCENTRATION
OF SOLID PARTICLE WASTES THROUGH
PACKED FLUIDIZED BEDS
Minal D. Pagedar and Dr. Rohinton K. Bhada
Dept. of Chemical Engineering, New Mexico State University
ABSTRACT
This article presents a study of the Fluidization of solid particles dispersed in densities and equal size distribution. This research was carried out with a potential to develop a novel process for preliminary concentration of mine tailings, a step into alternative economical environmental remediation. This initial study has utilized a surrogate binary solids mixture to represent mine tailings. The study resulted in a unique application of Fluidization technique for environmental remediation. Obtained results indicate a promising technology for solids separation without chemical treatment.
INTRODUCTION
Fluidization is an operation in which fine solids get transformed into a fluid like state through contact with a gas or liquid. With slight increase in the fluid velocity, the solid particles in the bed start to vibrate in a restricted region. The bed expands slightly and is called expanded bed. The spectrum of fluidization is divided into two areas: particulate fluidization and aggregative fluidization. Particulate fluidization is distinguished by the regular, continuous expansion of a bed of particles as the fluid velocity increases above minimum fluidization velocity to terminal falling velocity of particles. In aggregative fluidization, only limited expansion is observed as the fluidizing velocity is raised before some of the fluid begins to pass through the bed as bubbles.
Fluidized beds have been extensively modeled and studied for various applications like mass or heat transfer, mixing, elutriation and segregation. Until recently, fluidized beds had internal obstructions in bed called "bed internals" to break large bubble formation in order to enhance mass transfer and mixing. Hence fluidized beds with bed internals were studied with binary mixtures for good mixing and to avoid defluidization.
This research focuses on a unique application for fluidized bed containing packings for binary mixtures of solid particles. The system used in this research is a different density mixture where the density of both fractions varies significantly and the size of all the solid particles is the same.
In such a mixture, the fractions of particles of heavier density "jetsam" tend to sink relative to the other fraction of lighter density "flotsam." This is the Segregation phenomenon. Knowledge of segregation in a fluidized bed is important for several reasons. In most industrial applications, mixtures of solid particles of different densities are concentrated without any expensive separation techniques. For example a very useful application is for heavy metal concentration from mixtures of mine tailings for extraction. The heavy metals behave as "jetsam" while the lighter silica acts as "flotsam."
The advantage of this particular application is that it can be done without incurring additional expense to concentrate expensive metals without any standard metallurgical processing. The objective of this research was to show the feasibility of separation by density and to do a preliminary investigation of the optimum fluidizing time for the separation of the different density particles. Additionally the research determined the concentration profiles for each, the jetsam and the flotsam along the bed height. Studying a simulated mixture of two different density equal size solids by varying their mass ratios in the binary mixture completes this objective.
EXPERIMENTAL PROCEDURE
Apparatus
The fluidized bed was constructed from Plexiglass® tube 10 cm ID and 176 cm high. The tube was transparent and ½ inch in thickness. The bed had two sections: packed section and disengagement section. The packed bed had three sections flanged together. Each section was 10 cm ID and 20 cms long. Square, transparent plexiglass flanges were glued on either side with acrylic cement and had four bolt holes to hold three sections together. There was a rubber gasket in between each section for airtight column. There was a sampling port in the center of each section at 60 degree angle.
A small copper tube ¾ inch diameter with tyvex tubing worked as a sample port. An on-off valve was fitted on the tubing to open or close the sampling port. The top section was labeled as Section 1, middle section as Section 2 and the bottom section as Section 3. There was a small section at the bottom fitted with a stainless steel 425 mesh as an air distributor. The column was packed with Nutter® 0.7 rings.
The disengagement column was a single piece bolted on top of the packed section. The top of the disengagement column had an outlet for the withdrawal of fine solids gently. This occurs during the experiments when a floating layer develops at the top of the column and gets collected in the vacuum bag. The entire column was held by steel frame. The column was also grounded in order to prevent any hazards due to static generation. Air was the fluidizing medium and the flowrate was monitored by a Dwyer® rotameter. The pressure drop was measured at the bottom of column. The flowrate was maintained constant at 250 SCFM and the pressure drop was 40 psi.
Procedure
Various sets of experiments were carried out. These experiments were divided into two major categories: Fluidization with Nutter Packings and Fluidization without Nutter Packings (i.e., regular fluid bed operation). The binary solids mixture constituted of Glass particles (Si) and Iron particles (Fe). Solid particle size distribution was in very narrow range between 80 to 100 mesh.
During the experiments various mass ratios of two components were tried out for a binary mixture composition. This was incorporated with an emphasis to study the effect of volume and mass on the segregation in binary mixture.
Initially the column was packed with Nutter rings. Weighed amount of Fe powder was poured into the column. Equal quantity of Si powder was weighed and poured into column. This procedure was repeated till the entire column was packed, i.e., upto 66 cms. The air flow rate was set to a desired value (250 SCFM). The fine particles expanded from bottom layer, forming a particulate confined fluidized bed. Bubbles formed and broke around the packings and disappeared in the bed. At regular time intervals samples were withdrawn from all three sections while the bed was fluidizing. After each run was completed a slumped bed was also sampled. Withdrawn samples were weighed. A magnet was placed under a glass dish to separate the glass powder and iron powder from the withdrawn sample. The separated fractions were also weighed.
RESULTS & DISCUSSION
Experimental data collected over the entire research was plotted against several variables.
Variables studied in this research were the weight fractions of the components, average density of the sample, sampling height and sampling time interval. Volumetric flowrate and Pressure drop were constants here.
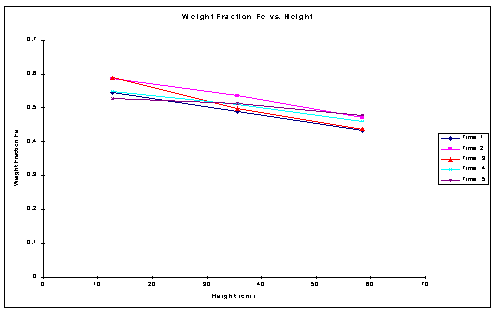
The following plot for the weight fraction (Fe) versus the height of the column shown in Figure 1. The samples withdrawn were for 1: 1 ratio for Si: Fe in the binary mixture. It can be observed that for fluidization with Nutter packings Fe particles segregate from the Si particles along the column height. The following Figure 2 shows the average density plot versus the height of the column.

Figure 1. Weight Fraction Fe (Xfe) Vs Height (h) for Si: Fe ratio of 1:1
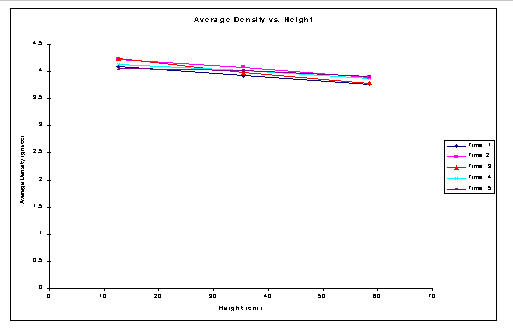
Figure 2. Average Density (r avg) Vs Height for Si:Fe ratio of 1:1
With increase in the height of the column the average density across the column decreases. The weight fraction of the Fe powder decreases with increase in the height. There was a distinct increase in the amount of Si powder with increase in the height. In order to confirm the same results two additional set of runs were performed. The obtained data and their plots confirm the above results.
The plot shows the segregation for Fe particles along the height of the column. The plots of the sampling height versus the weight fractions determine the characteristic separation along the height of the column. Similarly the average density profiles along the height were obtained. Plots of sampling time versus the weight fractions explain the undergoing dynamics of the system. Extended time runs indicated that the bed attained a steady state condition after certain interval of time elapses.
Figure 3 shows the comparative study of the Fluidization segregation obtained in the bed while fluidizing with the Nutter Packings and without the Nutter Packings. The dotted lines in figure 3 represent the experimental runs carried out without Nutter packings. The solid lines represent the data obtained for the runs with Nutter packings.
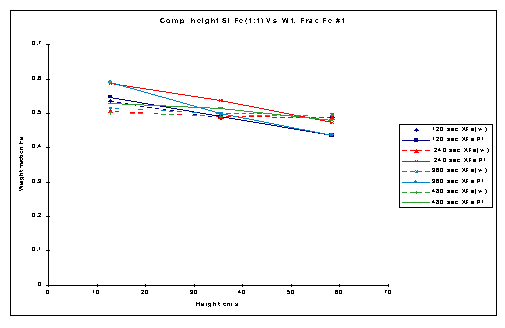
Figure 3. Comparison Plot of Weight fraction Fe Vs. Height for Si:Fe 1:1
The experiments carried out without the Nutter rings indicated an interesting set of results. The plots of these obtained results clearly pointed to complete mixing phenomenon in the absence of packings. No variation in average density across the length of the bed was found and it remained uniform throughout the entire bed height. The weight fraction of the Fe powder was same at all intervals in bed height. The amount of Si powder was same throughout the entire bed height.
Another important variable was time of fluidizing. The runs were carried out for a definite set of time at regular intervals of 2 minutes. The samples were withdrawn at every 2 minute interval while fluidizing. The average density on all three sections of the column showed a distinct increase between a time period of 200 to 360 seconds.
Discussion
For both the mass ratios’s Si: Fe (1:1) and (2:1) it was observed that the average density of the mixture doesn’t change along the bed height. There was uniformity observed over all the three sections of the bed.
It can be observed that in Fuidized beds without packings there was a complete mixing of the powders. There was no increase in the amounts of Si powder and Fe powder for both the ratios. However there was a slight increase in the amounts between interval of 200 to 500 seconds. Thus it indicated uniformity for the entire period.
It can be distinguished that there was definite pattern for both the mass ratios’ in the bed characteristics for experiments carried out with Nutter packings. For the ratio (1:1) the average density decreased with the increase in the bed height. This trend was uniform in all the three sections. For the ratio (2:1) the average density showed a sinusoidal behavior. Initially there is an increase then a decrease and again an increase, which levels off later. The mass ratio of Si: Fe (1:1) showed a definite decrease in weight fraction of Fe with increase in the height whereas there was an increase in the weight fraction of Si with increase in the bed height. Thus there was segregation of denser particles towards the bottom of the bed. The mass ratio (2:1) predicted a sinusoidal behavior for Fe. Similarly a totally reverse sinusoidal behavior could be observed for the Si. The amounts of both the mass fractions were more than the ones obtained from the same ports without packings.
CONCLUSIONS
For equal mass ratio of the flotsam and jetsam there was a distinctive pattern over the entire bed height indicating a definite separation of unequal density mixtures. The jetsam segregated near the bottom of the bed i.e. near the distributor plate whereas the lighter mass rises up in the column.
Bed internals aid in the initial mixing as well as eventual segregation of the particle mixtures based on their density variations and shape.
The bubble size reduces tremendously in the packed bed and presumably aids the separation process. It can be hypothesized that the small bubbles carry the particles in their wake and the heavier particles fall out whereas the lighter particles are carried over. In the mixtures of same size distribution the segregation thus, becomes the primarily function of the density.
RECOMMENDATIONS
Major focus of this research was feasibility study for the separation process of denser solid particles in a binary solids mixture. Packed Fluidized bed application with segregation of solid particles has not been implemented previously. The research indicates that separation of solid particles with same size distribution and varying density can be attained with a very high degree of efficiency for initial concentration purposes.
Due to time constraints many variables in this research were taken as constants. In order to identify major factors which effect segregation in a mixture like mine tailings would require additional experiments. Another set of experiments where fluidizing velocity and pressure drop are varied for various set of binary solids mixture ratio.
REFERENCES
ABBREVIATIONS
|
Xfe |
Mass fraction of Iron Powder |
|
Xsi |
Mass fraction of Glass Powder |
|
r avg |
Average density of Mixture |