THE FEASIBILITY OF USING CYANIDIUM CALDARIUM TO BIOREMEDIATE COPPER FROM ACID MINE DRAINAGE
Michelle H. Barragan and Sarah W. Harcum
Department of Chemical Engineering
New Mexico State University
Las Cruces, New Mexico
505-646-4145
ABSTRACT
Acid mine drainage is a concern in southern New Mexico due to its potential to contaminate the groundwater and surface water. This work investigated the feasibility of using viable Cyanidium caldarium to bioremediate copper from acid mine drainage in southern New Mexico. Copper was used as the model metal due to its toxicity and elevated levels in acid mine drainage in this area. The selected organism has been reported to remove heavy metals from aqueous solutions (Ahlf, 1988; Lucido, 1991; Wood and Wang, 1983). These authors did not fully investigate the effects of varying copper concentration, pH, CO2, and carbohydrate addition. This work investigated the effect of these parameters on copper removal from acid mine drainage.
INTRODUCTION
In southern New Mexico mining activities have greatly increased the flux of trace metals in the surface water. In particular, copper is found in very high concentrations (over 2,900 ppm) near old mine tailing sites that were abandoned near the turn-of-the-century. Additionally, copper concentrations are of special interest due to its strong affinity for organic ligands. Algae play an important role in the regulation and uptake of metals from aqueous system, therefore in cooperation with Phelps-Dodge in Tyrone, New Mexico, this research project was initiated to collect preliminary data on the feasibility of using C. caldarium for passive bioremediation of acid mine drainages near abandoned tailing sites that contain high copper levels.
C. caldarium has been reported to be able to grow in the presence of high metal concentrations by numerous authors (Ahlf, 1988; Lucido, 1991; Wood and Wang, 1983; Menefee, 1996). Also, three of these authors reported that C. caldarium could precipitate copper present in these aqueous solutions (Ahlf, 1988; Lucido, 1991; Wood and Wang, 1983). Wood and Wang (1983) and Ahlf (1988) also reported precipitation of copper from acid mine drainage. The copper concentrations used by Lucido (1991) and Wood and Wang (1983) are significantly lower than that found in the acid mine drainage of southern New Mexico. Therefore, this project investigated the feasibility of copper removal at copper concentrations more typical of southern New Mexico.
The objective of this study was to investigate the feasibility of using C. caldarium to selectively immobilize copper typically found in acid mine drainage in southern New Mexico. The project focused on the bioremediation of copper ions due to the high concentration of copper in New Mexico’s acid mine drainage. The culture used in this study is a mixed culture of C. caldarium and Galdieria sulphuraria, acidothermophilic red algae species. The culture used was predominately C. caldarium, therefore, the algae culture will be referred to as C. caldarium. Contacting parameters such as pH, initial copper concentration, and carbon source were varied to determine the effects of these parameters on copper removal. Results were obtained using viable biomass. Live cells were used due to the potential for in situ remediation.
MATERIALS AND METHODS
A mixed culture of C. caldarium and G. sulphuraria was graciously supplied by Dr. Richard Castenholz from the University of Oregon. The culture was collected from Nymph Creek, Yellowstone National Park. The culture is predominately C. caldarium, and will be referred to as C. caldarium in this work. The culture was grown in 20-L of media as described by Ford (1979), except that FeSO4•H2O was not chelated, the media was acidified with 1 N H2SO4, and the trace metal concentration was five-fold higher. The culture was bubbled with a humidified 6% CO2/air mixture at a rate of 2.1 L/min. The culture was illuminated by a 150 W halogen lamp supplying 800 foot-candles, which also provided excess heat. Stirring and temperature control were provided by a stir/hot-plate. The temperature was maintained between 45oC and 49oC. Growth was monitored at 520 nm.
The metal ion removal experiments used 0.5-L, 1-L, and 2-L volumes of culture broth. The harvested cells were centrifuged in 250 mL centrifuge bottles at 2,000 rpm for 10 minutes at 4oC in a Beckman J2-21 centrifuge. The supernatant was decanted. The centrifuged cells were re-suspended into fresh media at various pH’s. The copper was added to the flasks after the cells were re-suspended. The flasks were incubated in an incubator with fluorescent light at approximately 600 foot-candles at 45°C ± 1°C. Some experiments investigated the effects of sparging, 6% CO2/air mixture at a rate of 2.1 L/min in the contact media was used. Sterile glucose was added to the glucose addition studies.
Copper concentration samples, in duplicate, were taken and filtered through a 0.22 m m Whatman nylon filter to remove the cells. Copper analysis was conducted using a Perkin-Elmer 5000 atomic adsorption spectrophotometer. The filtrate was analyzed at a wavelength of 324.8 nm utilizing a copper lamp. When necessary, the filtrate was diluted to remain within the calibration range. A calibration curve was made for each set of samples using a CuSO4•5H2O solution as the standard in duplicate.
RESULTS AND DISCUSSION
The research of Wood and Wang (1983), Ahlf (1988), and Lucido et al. (1991) all demonstrated the removal of copper from aqueous solution mediated by live C. caldarium. This work attempted to repeat their results for copper concentrations more typical of southern New Mexico acid mine drainage. Parameters that were varied were copper concentration in contact media, contact pH, and the addition of carbon sources. Additionally, since C. caldarium has been reported to grow heterotrophically (Gross and Schnarrenberger, 1995), glucose was added to the contacting media to investigate the effect of glucose on copper removal.
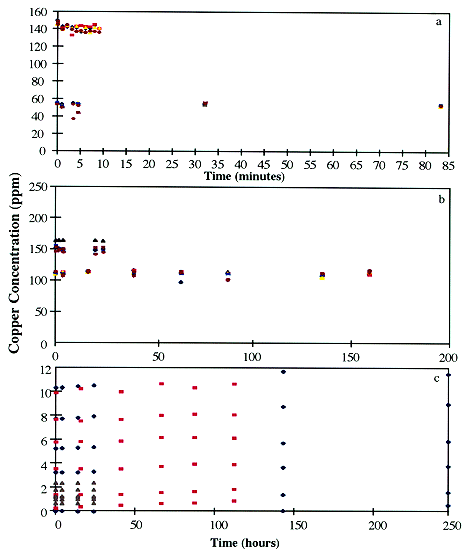
In the contact studies, the initial copper concentrations ranging from 0 to 200 ppm. The pH of the contacting media was varied from 1.8 to 7.0. Figure 1 shows the profiles of copper concentration in the contacting media with time. Figure 1a was an initial study to determine if the removal of the copper from the contact media occurred rapidly. The cells were contacted with 142 ppm copper at pH 1.8, 3, 4, 5, 6, and 7 and monitored for 10 minutes. No copper was removed by the biomass. Second, a longer experiment was conducted with an initial copper concentration of 55 ppm at pH 1.8, 3, 5, 6, and 7 and was monitored for 82 minutes. Copper was not removed by the cells for the longer experiment. These results indicate that the viable cells do not rapidly remove the copper from the solution.

Figure 1. Copper Concentration in Contacting Media with Time. a) Cells contacted at pH 1.8, 3, 4, 5, 6, and 7 with initial copper concentrations of 55 and 187 ppm. b) Cells contacted at pH 1.8, 3, 4, 5, 6, and 7 with initial copper concentrations of 107 and 142 ppm. c) Cells contacted with initial copper concentrations of 0, 1, 3, 5, 7, and 9 ppm at pH 1.8, 3, and 3.65. Also, cells contacted with initial copper concentrations of 0, 0.5, 0.8, 1.0, 1.5, and 2.0 ppm at pH 1.8.
The experiments shown in Figure 1b, investigate copper removal by C. caldarium for time periods on the order of hours. The first experiment used an initial copper concentration of 187 ppm at pH 1.8, 3, 5, 6, and 7 and monitored the copper concentration for 20 hours. No copper removal was observed. Next, an experiment with an initial copper concentration of 107 ppm at pH 1.8, 3, 5, 6, and 7 was investigated and monitored for 160 hours. No copper was removed from the contacting media.
The experiments shown in Figure 1a and 1b used relatively high initial copper concentrations (55 to 200 ppm), therefore lower concentrations of copper in the contacting media were investigated. The results shown in Figure 1c investigated relatively low initial copper concentrations. The initial copper concentration was reduced to 0, 1, 3, 5, 7, and 9 ppm and the pH of the contacting media was 1.8, 3.0, and 3.65 and monitored for 120 and 250 hours. A second experiment was conducted at initial copper concentrations of 0, 0.5, 0.8, 1.0, 1.5, and 2.0 ppm at pH 1.8 and monitored for 24 hours. For these low copper concentration studies, the only trend detected in the contacting media was a very slight increase in the copper concentration with time. This increase is possible due to the cells releasing copper into the contacting media that was sequestered from the growth media, which contained approximately 0.1 ppm copper, as an essential micronutrient. Viable C. caldarium did not remove copper from the contacting media in any of the conditions examined and shown in Figure 1.
Gross and Schnarrenberger (1995) reported that G. sulphuraria was able to grow under mixo- and heterotrophic conditions on 27 different sugars and sugar alcohols. It was reported that all carbon from the carbon sources, such as glucose, were used for cell growth. It was hypothesized that if the cells were supplied a surplus of carbon in the contacting media, the cells could grow and secrete/produce the compound(s) responsible for the metal binding. The additional CO2 would be incorporated into the cells by photosynthesis. The supplied glucose would be incorporated into the cell via the TCA cycle, thus making available the electrons generated by photosynthesis for metal binding, In Figure 2, the copper concentration of the contacting media with time for the sparged and glucose supplemented experiments are shown. Figure 2a depicts the concentration profiles of contact media at pH 1.8 (with and without sparging) and at pH 4 (with sparging). There was no significant amount of copper removed due to the CO2 sparged contact media.
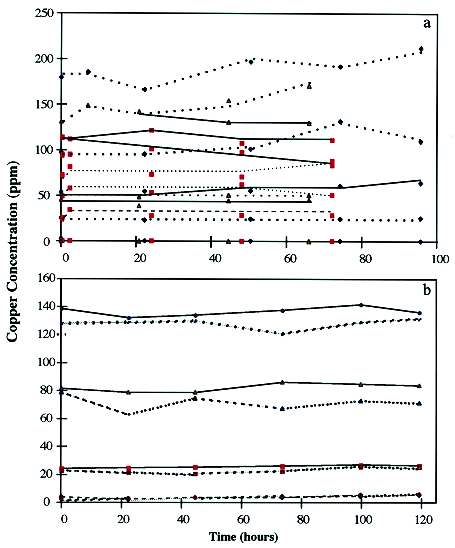
Figure 2. Copper Concentration in Contacting Media with Time. a)Cells contacted at pH 1.8 and 4 with initial copper concentrations of 0, 25, 50, 100, and 175 ppm with (dotted line) and without (solid line) sparging. b)Cells contacted at pH 1.8 with initial copper concentrations of approximately 0, 22, 80, and 130 ppm and glucose concentrations of 23mM (solid line) and 42 mM (dotted line).
Figure 2b shows the copper concentration profiles with time for two glucose additions (23 mM and 42 mM) to the contact media at pH 1.8. The initial copper concentrations were 0, 50, and 130 ppm. The addition of glucose to the contacting media did not enhance the copper removal by the cells, and no copper was removed. Therefore, the additional carbon sources did not mediate copper removal by the cells.
No significant copper ion removal was observed in this work despite the numerous parameters examined. This is contradictory to the copper removal reported for C. caldarium by other researchers. Possible reasons for the discrepancies are: the conditions described by the other researchers are missing key information, the C. caldarium mixed culture used in this work is significantly different from the pure C. caldarium strains previously examined, and the light spectra used by other researchers may have stimulated key surface proteins or functional groups that were not synthesized by the mixed C. caldarium culture. The previous authors did not describe the light source used, just the intensity. For this work, the growth culture was illuminated by one 150 W halogen light at approximately 800 ft-candles. Table I lists a summary of the conditions examined by this research project. Under none of the conditions examined, was any measurable copper removed.
Table I. Summary of the Contact Conditions Examined with Viable C. caldarium.
Note, copper was not removed from the contact media for any
of the examined conditions.
|
Harvest OD |
Harvest Volume (mL) re-suspended in 150 mL of media |
Initial Copper Concentrations (ppm) |
Growth pH at 45°C |
Contact pHs |
Sparged with CO2 |
Glucose Conc. in contact media (mM) |
|
2.15 |
500 |
55 |
1.8 |
1.8, 4, 5, 6, 7 |
no |
0 |
|
2.88 |
500 |
187.5 |
1.8 |
1.8, 4, 5, 6, 7 |
no |
0 |
|
1.6 |
500 |
107.1 |
1.8 |
1.8, 3, 4, 5, 6, 7 |
no |
0 |
|
2.57 |
500 |
142 |
1.8 |
1.8, 3, 4, 5, 6, 7 |
no |
0 |
|
1.75 |
500 |
0, 24, 47, 68, 88, 107 |
1.8 |
1.8 |
no |
0 |
|
1.7 |
500 |
0, 0.5, 0.8, 1.0, 1.5, 2.0 |
1.8 |
1.8 |
no |
0 |
|
1.4 |
250 |
0,1, 3, 5, 7, 9 |
1.8 |
1.8 |
no |
0 |
|
3.15* |
2000 |
0, 1, 3, 5, 7, 9 |
1.8 |
3.0 |
no |
0 |
|
2.36 |
1000 |
0, 1, 3, 5, 7, 9 |
3.0 |
3.2 |
yes |
0 |
|
9.65* |
500 |
0, 25, 50, 100 |
4.0 |
4.0 |
yes |
0 |
|
0.7 |
1000 |
0, 50, 130 |
1.8 |
1.8 |
no |
0 |
|
0.7 |
1000 |
0, 50, 130 |
1.8 |
1.8 |
yes |
0 |
|
1.7 |
500 |
0, 23, 80, 135 |
1.8 |
1.8 |
no |
23 |
|
1.7 |
500 |
0, 21, 82, 130 |
1.8 |
1.8 |
no |
42 |
*Denotes cells contacted at 34°C.
CONCLUSIONS
The objective of this research project was to determine the feasibility of using live biomass of C. caldarium to remove copper from acid mine drainage with the intent of developing a passive bioremediation system. It was anticipated that this system could have been implemented at turn-of-the-century sites like Deadman Creek, Tyrone, New Mexico located on Phelps Dodge property. This research project investigated the influence of numerous parameters on copper removal. Under all the conditions studied, using the viable C. caldarium mixed culture, copper was not removed. These results contradict those reported in the literature where pure viable C. caldarium cultures were used (Ahlf, 1988; Lucido, 1991; Wood and Wang, 1983). At this time, it is unclear why the mixed C. caldarium culture did not remove the copper from the contact media.
REFERENCES