GROUNDWATER REMEDIATION WITH PECAN
SHELLS AND CLAY
Jennifer Alwin and Dr. David Rockstraw
Department of Chemical Engineering
New Mexico State University
Las Cruces, NM 88003
ABSTRACT
Task II of the 1997 Waste-Management Education Research Consortium (WERC) Environmental Design Contest called for the remediation of a groundwater aquifer contaminated with Sr-90 and Cs-137. An interdisciplinary team from New Mexico State University developed and demonstrated a viable solution to this problem. The process design (i) minimized the volume of contaminated material requiring disposal, (ii) minimized worker intervention in the process, thereby reducing the potential for exposure, (iii) minimized the mass of process equipment requiring decommissioning at project completion, and (iv) developed a plan to gain public acceptance of the project.
During the process, contaminated groundwater is pumped through a series of 55-gallon drums containing an activated carbon and a pelletized clay. The activated carbon was developed from pecan shells, turning a current industrial waste into a product. The adsorbent materials removed the Sr-90 and Cs-137 from the water, with little interference from the natural groundwater components. The water is returned to the aquifer with radioactivity levels below regulatory standards. The waste, already packaged in disposal drums, is sent to the disposal site.
The process removed the contaminants, generated little secondary waste and eliminated human exposure. The design could be scaled up to process 100 million gallons of water at an operating cost of $0.06/gallon and a capital investment of approximately $2 million. Legal and regulatory requirements were identified and action taken to ensure compliance. An extensive community action plan was developed which included community involvement from project conception to completion.
INTRODUCTION
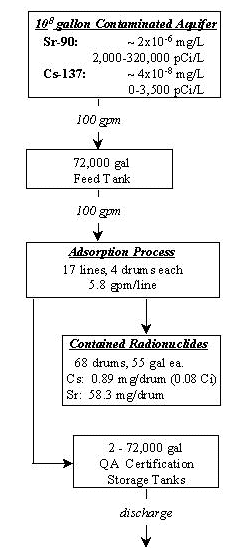
This paper describes a process designed to remediate an aquifer containing Cs-137 and Sr-90. The contaminant concentrations are relatively low (2x10-6 mg/L Sr-90and 4x10-8 mg/L Cs-137), when compared to the other components that are present in the water. This usually makes the isolation of these ions difficult, especially since most adsorbent materials show little ion selectivity. The task would require a large volume of adsorbent be used, problematic for most adsorption technologies when this application is considered.
Cs-137 and Sr-90 are both radioactive, but Cs-137 emits gamma photons. These emissions are regulated and the containment barrels cannot be transported if the dosage per barrel is in excess of the limits required by regulation. Significant expense is added for providing worker protection and meeting all regulatory requirements.
The design presented in this report has made a number of assumptions based on the given problem statement. The problem indicates a reservoir of 100 million gallons in volume. It was assumed that this is an absolute volume and that it is isolated from recharge/discharge dynamics. This assumption was made because information regarding type of recharge was not available. Information regarding the source of the contamination was also not made available from the problem statement. This is important since it is unknown whether or not the contamination comes from a point source. Because this information was not given, it was assumed that the contamination is homogeneous in the reservoir and the location of the well in relation to the contaminants is therefore unimportant. Since information regarding geological composition of the aquifer was not given, it is unknown how the contaminants might chemically bond to any sediment. The contaminants are thus considered to be completely dissolved in the groundwater. Ideally, the source of contamination would first be removed and then the contaminated water would be treated. Since this is unknown, this report focuses on the treatment of the contaminated water without addressing the source of contamination.

Figure 1.
TECHNOLOGY
The development of this process began by considering adsorption materials selective to Cs-137 and Sr-90. While other technologies were considered, the design chosen was based on the most efficient adsorption in the presence of other ion species. Certain clays were found to be effective in adsorbing the monovalent species such as Cs-137, sodium, and potassium. Finding a clay that selectively adsorbed Cs-137 allowed for a more efficient process. SMC was found to adsorb Cs-137 with a one to six ratio of Cs-137 over sodium(1). Sr-90 at low levels is difficult to remove from the water (Problem statement: 3x10-8 to 2x10-6 mg Sr-90/L). Additionally, interference by Mg2+ and Ca2+ ions was expected. Commercially available activated carbons are adequate, but expensive, and require regeneration. A new form of activated carbon (PS276bm) produced from pecan shells, was proven to be both inexpensive and extremely selective to Sr-90.
PS276bm Activated Carbon Preparation
PS276bm activated carbon is produced from the waste of the pecan industry. Pecan shells were obtained from processing plants in southern New Mexico. These shells were crushed and sieved to a particle size ranging from 1mm-75mm. Oxidation of the shells was carried out in a 2-liter batch reactor. Activation was achieved by mixing an amount of shells with an equal mass of an acid solution. The mixture was heated to a moderate temperature while agitating in the presence of an air sparge. The resulting carbon was cooled to room temperature, washed with distilled water and soaked in a dilute sulfuric acid for thirty minutes. The carbon was then dried and screened. The result was the production of an activated carbon that costs $0.04/lb compared to the average cost of a commercially prepared activated carbon at $0.26/lb. Adsorption of PS276bm is discussed in the Technical Assessments section of the report.
Sodium Monmorillinite Preparation
Samples of untreated sodium monmorillinite clay (SMC) were obtained and cleaned prior to use. The SMC was suspended in water and stirred for approximately 15 minutes. The solution was allowed to settle for 24 hours. The SMC remained suspended in the water and was removed by decanting. The SMC was then isolated by vacuum filtration. Adsorption of the SMC is discussed in the Technical Assessments section of the report.
PROCESS DESIGN
Overview
The process system for the removal of Cs-137 and Sr-90 from groundwater is designed to be economical and require as little human intervention as possible, thereby limiting potential exposure. Water is pumped to a 72,000 gallon tank using a standard water well lift pump (2). The water is then distributed from the tank by a ˝hp centrifugal pump to a manifold system consisting of seventeen identical lines. Each line contains five 55-gallon drums in series. There are also be two standby lines to be used in case of an emergency. The drums are packed with the adsorption materials (PS276bm and SMC). The water passes through the drums at 5.8 gpm per line to one of two final storage tanks (3). The two product tanks will allow for certification of the water purity by gross beta/gamma analysis prior to discharge. If radiological testing is acceptable, the water is then pumped back into the upstream end of the aquifer forcing a concentration gradient in favor of the process.
Lift and Storage
The groundwater is pumped from the aquifer to a storage tank via a submersible pump. The storage tank is designed to give the primary system twelve hours of operation in the event that the well pump is shut down, or to provide storage for water which may be re-circulated from the main treatment process. This tank also operates as a buffer for fluctuations in the flow rate and concentration from the well.
Contaminant Removal
The contaminated water from the feed tank is pumped to a manifold system which distributes the water to seventeen identical processing lines. These lines each contain five 55-gallon drums. The first drum in each process line contains PS276bm activated carbon. The second and third drums in the process lines are filled with pelletized SMC. The fourth and fifth barrels are included as over-design or as finishing filters. Radioactivity levels must be less than 4x10-9 mg/L for Sr-90 and 1x10-8 mg/L for Cs-137 in accordance with 40 CFR 131 and 10 CFR 20.
The design of the manifold system is based upon the process flow rate and the limit on the amount of Cs-137 that can be adsorbed before dose limits at the drum surface are exceeded. Approximately ten million gallons of water can pass through any single line, with 0.899 mg of Cs-137 adsorbed, before shielding is required (4). Seventeen lines, each containing two barrels of SMC in each line are used to guarantee that the container dosage requirement is not exceeded. To further ensure worker safety, the primary SMC barrel in each line contains 2 cm of lead shielding. A difficulty encountered in using SMC as an adsorption media is the small particle size. A pelletized clay is used to limit suspension of particles and pressure drop through the barrel. Each drum used for adsorption of Cs-137 will be packed with approximately 950 lb of pelletized SMC.
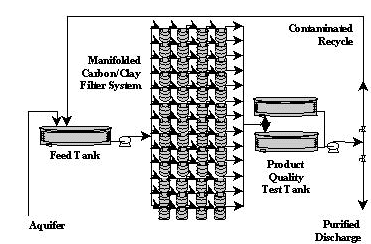
Figure 2. Process Flow Schematic
PS276bm activated carbon is used to remove the Sr-90 from the contaminated waste stream. This particular activated carbon is extremely selective toward Sr-90, showing little interference from other divalent ions. Preliminary calculations indicate that the Sr-90 present in the aquifer could be removed using 1 gram of PS276bm.. Each barrel used for Sr-90 adsorption contains approximately 190 lb of PS276bm. The system has been over-designed to account for possible changes in solution equilibria as the concentration of the treated water changes. Dynamic flow studies on the phenomenon will be necessary to determine the effect of the changing solution concentration on adsorption equilibria.
Design of Adsorbent-Containment Drums
To prevent water from flowing from one bunghole directly to the other, short-circuiting the adsorption bed, a pipe will be installed to the effluent bunghole as illustrated in Figure 3, forcing the water to pass across the entire adsorption bed. This pipe will extend to the top of the inverted drum to cause maximum saturation of the bed. The drums will be inverted and suspended on a rack to aid in liquid removal during final preparation for disposal.
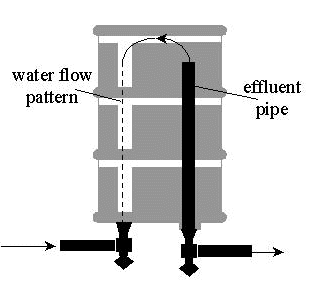
Figure 3. Modified 55-gallon Drum
Sampling, Breakthrough, & Emergency Procedures
The system is designed to treat 100 million gallons of water without breakthrough of Cs-137 or Sr-90 from the first three barrels in any line. Sampling will be performed on each line on a regular basis to insure that no contaminate is leaving the process and entering the storage tanks. Operators will sample three lines each hour. Thus, each line will be sampled approximately every six hours. Samples will be tested using inductively coupled plasma analysis (ICP). In the event breakthrough is detected between drums three and four, that line will be removed from operation and a standby line will be placed in service. The treated water in the certification tank will then be tested for contamination by gross beta/gamma analysis. If contamination is detected in the product certification tank, the water in that tank will be re-circulated to the feed tank.
To certify water discharged back into the aquifer is clean, the product tank will be sampled and tested using gross beta/gamma analysis. If the water in the tank passes the certification, it will be discharged back into the aquifer. If the water quality is found to be unacceptable, radiochemical analysis for the Sr-90 and gamma spec for Cs-137 will be checked. It the water fails this test also, it will be re-circulated into the feed tank. To maintain a continuous flow system one quality assurance tank will be tested while the other one is being filled.
Final Disposal Preparation
Before the barrels can be shipped to the disposal site, the water must be drained to less than one inch at the bottom of each drum. The barrels will be held inverted on a rack allowing them to be drained by gravity and an air purge.
Any water drained from the barrels will be re-circulated into the feed tank. Once the barrels are sufficiently dry they will be sealed. Radiation surveys will be performed on groups of drums to ensure the surface dose rate of the group will not exceed shipping limits once placed on the truck. The waste will be shipped to the final disposal site. .
ASSESSMENT
Technical Assessments
The process has been designed using the latest developments in adsorption technology, combined with a relatively simple process design. Each 55-gallon drum acts as a miniature adsorption column, removing the contaminants, while providing containment, storage, and a disposal media for the removed species.
The selectivity of the carbon and the clay makes the system adaptable to many remediation sites. The number of barrels and lines may be altered to meet the volume and concentrations of other aquifers. At present, there is insufficient data on spent PS276bm to assess whether immobilization of the final waste form is needed. Preliminary data indicates that the Sr-90 precipitates as SrSO4, which is insoluble in water and thus highly immobile. If immobilization is found to be necessary, it can be accomplished by a number of methods (in-situ polymerization, concrete addition, vitrification, etc.).
Shielding requirements for this process are minimal. The first SMC drum (adsorbing gamma-emitting cesium) in each line will have about 2 cm of lead as a precaution (5,6). Simulations using MicroShieldŽ software show the adsorbent materials provided sufficient shielding such that no additional shielding would be necessary. The calculated value for shielding was based on the assumption that the Cs-137 is homogeneously distributed in the drum. As standard operating procedure, routine radioactivity surveys will be performed to verify actual radiation levels at the drum surfaces during processing. In addition, a survey of dosage level will be performed on the shipping truck once the drums have been loaded for transportation to ensure that dosage measured on the outside of the truck does not exceed regulatory limits.
The experimental nature of the adsorbent material will require further study before the process can be applied. It is unknown how changes in the pH or composition of the aquifer will effect the adsorptivity of the PS276bm and SMC. It is possible that shifts in pH will occur as the water is processed through the bed (7).This could effect the kinetics of the adsorption process. Since the Mg and Ca are not adsorbed by the PS276bm, the ionic nature of the water is not expected to be significantly impacted by the separation. The presence of organic contaminants in the water could potentially poison the PS276bm or SMC and prevent the desired adsorption of Sr-90 and Cs-137 respectively, but no data on the effect of these species is available.
Adsorption isotherms for Sr and Cs on PS276bm can be found in literature to be published this year. Studies indicate a precipitation phenomena of the Sr onto the PS276bm, with the Sr likely precipitating as SrSO4. Equilibrium data for adsorption of Cs and Sr onto SMC reveal that SMC has a good adsorption affinity for Cs (1). The adsorption isotherm with SMC shows a maximum of 100 mg Cs/g SMC.
Economic Assessments
Capital costs were estimated from uninstalled equipment costs, using Lang factors for a fluid processing plant (8). Factors for costs associated with implementing the community action plan and permitting were included to provide a more realistic "bottom line." The cost for adsorbents are $0.04/lb PS276bm and $0.10/lb SMC (9). The 55-gallon drums will require modification to be used for radioactive materials and to allow connectivity in the process. Drum price was estimated at $500 each by consideration of cost of materials, construction, inspection, and certification.
Table I. Economic Assessment

The most significant costs of the process are the operating costs of the facility. Much of this is due to laboratory costs associated with analytical services, including on-site radiochemistry analysis capability. Manpower costs were estimated for continuous operation, with a labor rate of $21/hr for four operators and $40/hr for supervision. Operating costs were calculated assuming that 108 gallons of water are treated. At a rate of 100 gal/min, the time required to process the entire volume is slightly longer than two years. The aquifer will show a drop in Cs-137 and Sr-90 concentrations as the treated water is re-injected into the aquifer, though dilution will be minimized by the location of this reintroduction. The cost per gallon of water treated will be approximately $0.06/gallon.
Decontamination/Decommissioning
Decontamination/decommissioning of the site must be included in a realistic cost estimate. An estimate was obtained based on area requiring decontamination. Approximately 104 ft2 of site will need to be decommissioned after the water has been treated. A cost of $200/ft2 was assumed to include decommissioning in the total project cost.
The final waste form will be transported to a low-level waste (LLW) site. It is estimated that it will cost approximately $5,000 per drum for transfer of the contaminated adsorbent drums to the LLW facility. This results in a total disposal cost for the drums of $425,000.
BUSINESS PLAN TO ENSURE LEGAL, HEALTH AND
REGULATORY COMPLIANCE
Documentation & Permitting Regulations
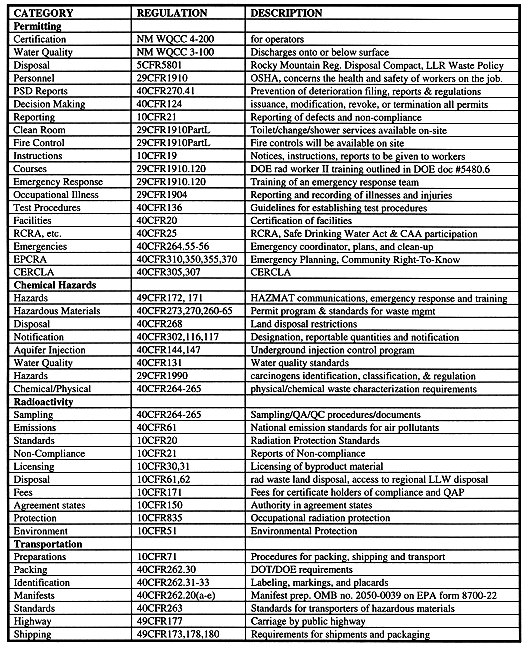
The process design for the removal of contaminants from an aquifer will be operated under the principles of Process Safety Management (PSM) in accordance with 29 CFR 1910.119. The PSM regulations are performance-based, requiring complete documentation of operations, including: (i) start-up, shutdown, normal and emergency operating procedures, (ii) a chemical hygiene plan, (iii) a respiratory protection plan, (iv) a hazard communication program, and (v) a management of change program. All employees will have access to these and other plans, programs and information, and will receive training in the above. Process hazards reviews using Fault Tree Analysis and Hazards and Operability (HAZOP)studies will be completed (10,11,12,13).
The process permitting is regulated under titles 10, 29 and 40 of the Code of Federal Regulations. The entire area will be a secured area and will be enclosed on all perimeters by fence. The operation will be certified under the state of New Mexico Water Quality Control Commission Regulations. Local regulations for certification and water quality are satisfied by state regulations. Federal regulations for water quality will be met in accordance with 40 CFR sections 25, 144, 147 and 131.
New Mexico is a member state of the Rocky Mountain Region Disposal Compact. The Rocky Mountain Board has a contract with the Northwest Interstate Compact Committee and the State of Washington for disposal at the Northwest Compact's regional commercial disposal facility in Hanford, Washington. The LLW generated will be shipped to Hanford under this compact.
Chemical Hazards Regulations
The materials that are used in the process have low chemical hazards relative to the radioactive hazards. All materials are chemically stable under normal operating conditions. The chemical hazards of these materials are covered by compliance with the regulations for radioactivity hazards found in the next section.
Radioactivity Hazards Regulations
With the intent of the process to reintroduce the cleaned water back into the aquifer, the process must follow 10 CFR, Appendix B, 20.1-20.601, as well as the state and local regulations concerning water quality control. This region is regulated by the State of New Mexico Water Quality Control Commission. If the process is performed at a DOE site, 10 CFR 835 must be followed, while release limits would be regulated by 10 CFR 834.
All OSHA regulations will be followed and the safety of operators and the protection of the environment will be the highest priority. PSM standards, EPCRA and Code of Federal Regulations will be followed for accidents and emergencies. These will be reported to U.S. Nuclear Regulatory Commission immediately under 10 CFR 30.5.
Department of Transportation (DOT) Regulations
All federal, state, and local regulations regarding packaging, transportation and labeling regulations will be met. The certification and date for the waste will be sent to the disposal facility and receive approval before the waste is ever transported. This will ensure that waste that is shipped will have a destination.
Table II. Regulations
COMMUNITY ACTION PLAN
In this day where hazardous materials are commonplace, remediation processes are a necessity. Many different technologies exist, or are being created to accomplish various cleanup tasks. However, no technology, regardless of effectiveness, can do the job if it cannot be implemented. Implementation of a technology is dependent upon two factors; money and cooperation from stake holders.
One of the main risk drivers for all cleanup projects is implementation hold-ups due to public outcry. Contract jobs by nature must adhere to the time table used to calculate costs, in order to yield the expected cash flows. When jobs are interrupted, or are stopped before they begin, fixed costs continue to pile up, while no work is accomplished. Prevention of public outcry and interference logically becomes the next key point.
Public involvement and ownership of the project is the best way to not only guard against interference, but assure assistance in accomplishing the cleanup task. "Ownership" in that statement refers to a general feeling by the stake holders that the technology being used is not only in their best interests, but that it is in part their accomplishment.
Many different entities make up the collage that is identified as "stake holders" and "the public". Governments, including local, municipal, federal, tribal and local interest groups need to be approached in different manners. All areas affected by the cleanup process must be included in the identification stage. This applies to not only the cleanup site, but to transportation routes, and disposal areas. To give a more cohesive overview of stake holder identification and interaction, those groups previously mentioned will be addressed individually.
Governmental Relations
Awareness of applicable legislation, permit requirements, and standards for cleanup is vital. The process of clearing through the red tape should be done by a professional who is both knowledgeable, and adept at dealing with the Federal Government, specifically the EPA. In many instances State requirements exceed Federal, and must be addressed. An experienced environmental lawyer should be employed to ensure compliance. Local Government is one of the best sources of initial public involvement for any operation. Local politicians tend to know their constituents, and are able to lead them, to a certain extent, in the direction of understanding and cooperation with a cleanup proposal. Obtaining the names of groups and individuals who will hold the most potential for assistance, as well as hindrance, is often the easiest through local politicians.
Tribal Relations
Cultural differences between Native American tribes and entities implementing remediation action which takes place on or requires transport through tribal land, can lead to confidence breaking mistakes done out of ignorance. An individual who has an intimate knowledge of the tribe in question must educate all those coming in contact with the tribal members and representatives as to the proper ways to interact. A respected tribal member should be employed as a liaison for that purpose. Ideally, one or more tribal members who have qualifications in either the business or technology realms should be considered for employment in the design and implementation process.
Interest Group Involvement and Public Interaction
Project planners cannot expect to simply let public interest groups know what they are doing, and expect to receive no resistance. Interest groups must be involved in the design process to ensure all goes smoothly. Since roughly 70% of project costs are incurred in the design stage, a technology not understood or immediately liked by such groups will probably never see the light of day. Indeed, simply being told that even the best possible technology is already slated for implementation will cause some groups to balk and set out to prevent the startup of the principle.
Going to interest groups to talk about the problem that needs to be addressed, and inviting them to make suggestions, and identify specifications for the cleanup if the first step. Contacting these groups can be done by asking to speak at luncheons, and attending public meetings such as city council and school board meetings to talk briefly about the task at hand. Interested individuals should then be given the opportunity to form an advisory committee to work closely with design engineers. Going to the public and eliciting opinion and exchanging information is the only sound method of public involvement.
Addressing these concerns, and outlining a plan for accomplishing the large tasks of governmental and tribal relations, and public involvement is the best way to ensure the success of the project. This will inevitably provide credibility in the eyes of agencies or businesses reviewing bids for research and eventual implementation of remediation processes, making implementation possible.
CONCLUSIONS
The system presented in this report is an efficient and economical approach to the problem of Cs-137 and Sr-90 removal from groundwater. The process has been designed to meet all pertinent regulations and requires a minimum amount of user intervention. The technology is simple, effective and readily available. The treatment cost per gallon of water is $0.06/gallon ($5.8 million total) with capital costs of about $2.8 million. Disposal costs associated with the final waste form and decommissioning are estimated to be $425,000 and $2,000,000 respectively.
REFERENCES
ACKNOWLEDGEMENTS
The New Mexico State University team was guided under the direction of Dr. David A. Rockstraw, NMSU Chemical Engineering Professor. The team members: Jennifer Alwin, Sean Barley, Isela Ceballos, Kevin Chapman, Jessica Chavez, John Corpening, Joe Delzell, Gary Deselle, Jennifer Griffin, Liz Holland, Dev Kambhampati, Renee LeGendre, Maylayne Mahar, Minal Pagedar, S. Rajagopalan, Dirk Sanderson, Reyad Shawabkeh, Vernon Solis, Scott Underwood, Ryan Van Pelt, Fujie Zhang, are all responsible to the development of this design.
Members of the team acknowledge sincere gratitude for the assistance of the following individuals: Tom Goff, Westinghouse, Waste Isolation Division, Dr. Richard Long, New Mexico State University, Chemical Engineering, Al Trujillo, Global Advanced Technologies, Dr. Adrian T. Hanson, New Mexico State University, Civil Engineering, Dr. Zohrab A. Samani, New Mexico State University, Civil Engineering, Dr. Antonio S. Lara, New Mexico State University, Chemistry, Dr. Ron Bhada, New Mexico State University, Chemical Engineering, Steve Salopeck, Sun Diamond Pecan Growers.