IMMOBILIZATION OF SALT-CONTAINING SURROGATE
MIXED WASTES USING POLYESTER RESINS
Rabindra K. Biyani, P.E., Douglas W. Hendrickson, P.E., and Ted M. Hohl
SGN Eurisys Services Corporation
ABSTRACT
This paper presents the laboratory test results of microencapsulation of salt-containing surrogate mixed wastes using polyester resins. With U.S. Department of Energy (DOE) funding, three waste types, at varying loadings, were encapsulated in four different resins. Two waste types tested were solid surrogates representing the majority of DOE mixed salt wastes. The third waste type, an aqueous slurry, represents waste from DOE effluent treatment plants.
Waste form specimens, prepared by curing a mixture of resin and salt waste, were subjected to compression, static leach, TCLP (Toxicity Characteristic Leaching Procedure), and water immersion performance tests. Results show that 50 weight percent dry salt waste loading can be achieved in polyester waste forms. The maximum feasible waste loading varied with the physical nature of the waste.
TCLP specimens prepared by attrition yielded failure in leachate cadmium concentration. However, when a specimen was prepared by a revised and simplified method, contaminant concentrations were below the TCLP limits. Compression strength and static leach test requirements were easily met. Overall, a viable method of salt waste stabilization has been demonstrated.
INTRODUCTION
In mid-1997, the U.S. Department of Energy (DOE) (EM-50), through its Mixed Waste Focus Area (MWFA), funded an evaluation of the capability of polyester resins to immobilize wastes that represent salt-containing mixed wastes generated or being generated at DOE facilities. Results from surrogate waste tests performed since July 1997 are presented here.
The overall objective of these tests was to demonstrate, via factorially designed bench-scale tests, the treatment of surrogate wastes by encapsulation into four different polyester resins, one being a vinyl ester resin. The MWFA specified two salt-waste compositions to be tested, one containing high chloride (~10 wt.% NaCl), the other high nitrate (~ 60 wt.% NaNO3). A third waste type tested was an aqueous slurry containing 25 wt.% salts approximately one-fifth of which were undissolved sulfate salts. This waste represented a high-volume secondary waste stream from the Effluent Treatment Facility (ETF) in the 200 East Area at Hanford, Washington.
DESCRIPTION OF SURROGATE WASTES TESTING
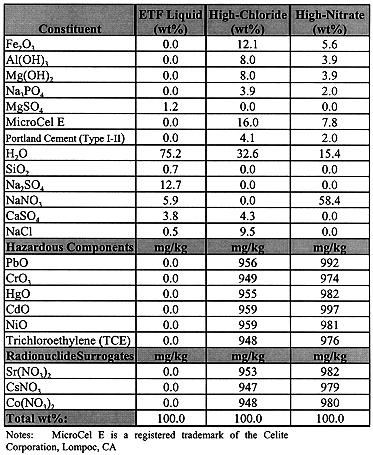
The three waste simulants (Table I) selected for testing were prepared at the Standards Laboratory in the 200 West Area at Hanford. The liquid waste simulant was based on the ETF evaporator bottoms stream resulting from contaminated groundwater treatment. The ETF treats different types of contaminated waste waters. The final step in the ETF secondary treatment train is a thin-film drier, which has experienced frequent operational problems. As an alternative to chemical pretreatment and processing in the drier, the evaporator concentrates could be directly microencapsulated with a polyester resin.
Table I. Composition of Mixed Waste Test Simulants

The particulate waste constituents specified by the MWFA were ground in a planetary mill to ensure homogeneity. Once the solids were blended, the liquid constituents, trichloroethylene (TCE) and water, were added and mixed in with a powered stirrer. The initial grinding of solids dramatically increased the bulk volume of the waste. The bulk density of the chloride and the nitrate wastes was 0.42 and 0.68 g/mL, respectively.
The solid phase wastes were encapsulated using an orthophthalic polyester resin (AropolÔ S2293); an isophthalic polyester resin (AropolÔ 7334), and a vinyl ester resin (HetronÒ 922-L25). The performance of these resins was expected to improve in the following order ¾ orthophthalic and isophthalic polyester resins, followed by vinyl ester resins. The resin used for aqueous waste encapsulation is AropolÔ * WEP-662P, a water-extendible polyester (WEP) resin. All resins procured (5) contained promoters to facilitate room temperature curing.
Used widely in the manufacture of fiberglass-reinforced plastic (FRP) products, polyester resins are cured by free-radical polymerization started by adding an initiator, e.g., methyl ethyl ketone peroxide. Polyester resins contain 40 wt.% - 60 wt.% styrene monomer, which provides crosslinking to form a rigid three-dimensional polyester matrix. The quantity of initiator added and the initial temperature of the ingredients used are the main variables in determining gel time of prepromoted resins. Specimen preparation temperature, which affects the curing rate, was kept ambient throughout the tests.
Vinyl ester resins, though broadly classified as polyesters, are typically diesters that contain repeating ester linkages. Both vinyl esters and polyesters have been used commercially for the solidification of radioactive and hazardous wastes (6). Vinyl ester resins, with a lower ester content, may be more resistant to hydrolysis by water than other polyester resins. However, with greater unsaturation in the polyester backbone, the frequent polystyrene linkages in cured polyester resins hold together the cross-linked structure like a wire mesh grid. Excellent retention of contaminants in cured polyester resin has been proven by leaching tests (6, 7).
Design of Experiments
Three primary factors were explored in these tests: resin type, waste type, and waste loading. Initial experiments with the two solid phase simulants were aimed at determining the upper limit of waste loading into the resin. At 60 wt.% chloride waste, it was discovered that the resin waste mixture became very viscous and difficult to mix. It was conservatively estimated that 50 wt.% loading would be the feasible upper limit in full-scale processing. The preliminary tests also enabled determination of suitable initiator ratios for curing each resin waste mixture. After this exploratory study, initiator ratios were fixed for the remainder of testing (Table II).
Table II. Physical Data and Run Averages by Resin Type
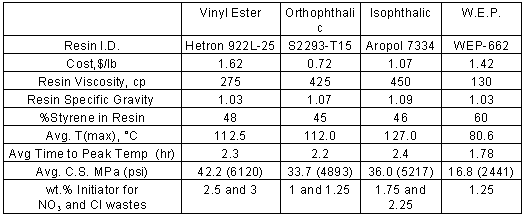
The factorial study of variables was performed at 40 and 50 wt.% waste loading and spanned a 3 x 2 x 2 factorial experiment design. This experiment design allowed the three primary factors to be explored over a range potentially useful for actual waste processing.
Tests with the 25 wt.% solids ETF waste are tests separate from the solid phase mixed waste simulants. The main purpose for testing the aqueous phase simulant was to demonstrate the feasibility of using a WEP resin to successfully encapsulate an aqueous phase mixed waste.
Specimen Preparation
The solid phase waste immobilization process consisted of first blending a predetermined amount of room temperature polymerization initiator into a batch of polyester resin in a mixing vessel. The free-flowing salt wastes were then added to the initiated resin and intimately mixed. If proper mixing is achieved, the resin coats each waste particle then cures forming a hardened waste monolith.
The synthetic chloride waste, being more voluminous than the nitrate waste, was difficult to mix and pour into molds. It was powdery and dry despite being 32.6 wt.% water. It was transferred to the mixing bowl in steady scoopfuls while mixing continued. The speed of the beater mixing blade at 96 rpm, with close clearances over the sides and bottom of the mixing bowl, gave rapid dispersion of the waste throughout the mixture. Before filling the molds, the waste resin mixture was finally mixed with a spatula to improve the uniformity of the mix. The filled molds were capped and placed in an adiabatic chamber for resin curing. A thermocouple, tightly sheathed with aluminum foil, was positioned at the center of a 200-mL mold for each run and the rise in temperature during resin curing was recorded via a datalogger.
For the aqueous waste, a special WEP resin was used to make a waste-in-resin emulsion. The resin phase was then cured by initiator addition, which resulted in a monolithic waste form in which the waste was immobilized as micron-size droplets within closed cells of the resin. A batch of the WEP resin was mixed by a high-shear blender as the slurried waste was added in a steady stream. Approximately five minutes of mixing at the "low"speed setting produced a stable waste-in-resin emulsion. The initiator was then added and mixing continued for two minutes. The initiated emulsion was poured into molds and the filled molds were placed in the adiabatic curing chamber. Rise in temperature during resin curing for both aqueous and solid phase wastes indicated proper hardening.
TCLP samples were prepared from waste form fragments created during compression testing. In all cases these fragments were large and, per the TCLP test procedure, had to be reduced in size to less than 1 cm in their narrowest dimension. The rigidity of the solidified waste form made it very difficult to achieve this. Sawing of the samples generated considerable dust and was abandoned. In its place, a bolt cutter was used successfully to prepare samples for TCLP testing and zero head extraction (ZHE) for the volatile analytes. Immediately after the pieces met the size criteria, they were placed in sealed bottles to prevent loss of the volatile TCE.
Preparation of the six TCLP samples required size reduction of waste forms by attrition or shearing. This action generally destroyed the resin encasing of the newly created surfaces. The resulting exposure of the otherwise encapsulated waste particles contributed to contaminant leaching. To resolve this problem, researchers have used a specialized mold to prepare specimen pellets sized to meet the TCLP sample-size criteria (8). To evaluate true leaching characteristics, one TCLP test was repeated in which this simplified sample preparation method was used.
TEST RESULTS
The solidified waste form specimens were subjected to qualification tests (1, 2, 3, and 4) per Environmental Protection Agency (EPA) and Nuclear Regulatory Commission (NRC) approved methods. These included waste form morphological observations, compressive strength measurement, TCLP and static leach testing, and a water immersion test.
Physical Observations
No free liquids were observed in any of the samples. The surfaces of most samples were smooth and tack-free. Exceptions were samples from the run that exhibited the highest peak curing temperature (147.7°C). The rapid heat generation, by the isophathalic resin in this run, created stresses within the waste form samples. This resulted in some minor surface cracks containing salt deposits, possibly from the diffusion of water vapor formed when the temperature exceeded 100°C. However, the overall integrity of those waste forms was demonstrated by specimen compressive strength reaching over 34.4 MPa (5000 psi), which exceeded the average compressive strength (Table II) of 33.7 MPa (4893 psi) for this resin. The water immersion sample from this batch remained intact after 90 days with no change in the sample cracks at the end of the test.
For the solid phase waste, the specific gravity of waste forms ranged from 1.27 to 1.55 while for the liquid phase waste form at 60 wt.% loading the specific gravity was 1.17. The volume reduction factor, defined as the ratio of raw waste volume to the waste form volume, ranged from 0.79 to 1.93 for the solid phase waste. The average volume reduction factor for this waste was 1.34, implying a 25 percent reduction of the raw waste volume. A volume reduction is possible as the resin fills up only the void spaces and facilitates settling and compaction of the bulk particulate waste.
For the ETF slurry the ratio of raw waste volume to the waste form volume was 0.61, implying a 50 percent increase of the raw waste volume. Table II presents data on the physical properties of the resin and a synopsis of its average peak temperature and the corresponding time to peak exotherm.
The peak curing temperature and the associated time to reach this are depicted in Fig. 1 and Fig. 2, respectively. As expected, for the same resin, the runs with higher (50 wt.%) waste loading exhibited a lower peak temperature than runs with a lower (40 wt.%) loading and the higher peak temperature was reached in less time. Lower waste content meant a higher resin amount, which upon polymerizing, would generate more heat and raise the peak temperature. The average peak temperature was lowest for the orthophthalic resin.
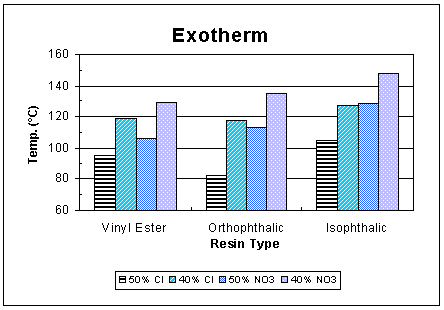
Fig. 1. Peak curing temperature.
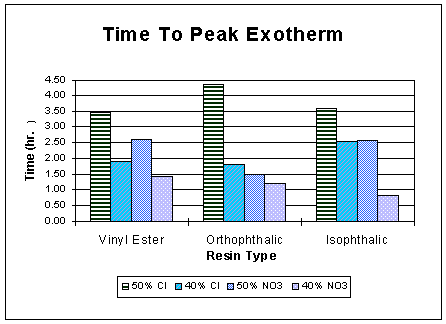
Fig. 2. Time to reach peak exotherm.
For particulate wastes with substantial water, peak resin curing temperatures higher than 100°C should be avoided due to an increased likelihood of waste disposal container pressurization. To reduce the rate of polymerization and concurrently slow the heat generation, the orthophthalic resin was modified by the resin vendor. The reformulated polyester backbone was based on dicyclopentadiene (DCPT), which provided unsaturation sites for crosslinking.
Salt waste specimens were made with two such reformulated polyester resins identified as S5646A and S5646B. Waste loading was at 40 wt.% chloride waste and the exotherms were monitored with a thermocouple and datalogger. When previously run with S2293 orthophthalic resin, the waste form reached a peak temperature of 117.3°C (in 1.8 hours). Similar waste forms prepared with the revised resins S5646A and S5646B reached 107.2°C (in 4.9 hours) and 100.8°C (in 7.0 hours), respectively. The new resin S5646B offered a 16.5°C reduction in peak exotherm. This simple test proved that exotherm reduction is feasible with little difficulty.
Compressive Strength Tests
Compressive strength measurement was performed on one 200-mL sample from each run using the American Society of Testing and Materials (ASTM) C 39-93a procedure (1). The samples were capped with a sulfur-based capping compound to ensure planed surfaces. Compressive strength results of the solid phase waste samples are presented in Fig. 3. Compressive strength ranged from 25 MPa (3800 psi) to 47 MPa (6800 psi) which is indicative of proper resin curing. The compressive strengths of the liquid phase samples was 24 MPa (3400 psi) and 10 MPa (1400 psi) for 60 and 70 wt.% waste loading, respectively. Samples from dry waste runs surpassed the minimum requirement of 0.4 MPa (60 psi) by a factor of at least 60 and the factor for liquid waste was at least 23.
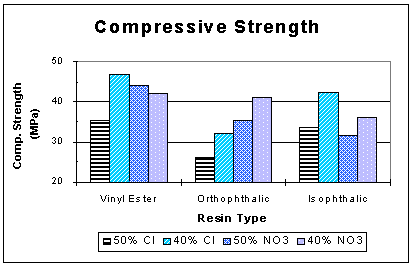
Fig. 3. Compressive strength results.
Modified and Abbreviated ANSI/ANS 16.1 Static Leaching Test
A modified and abbreviated ANSI/ANS 16.1 static leach test (2) was performed on 14 samples made with the vinyl ester and orthophthalic resins. These resins had thus far performed better than the isophthalic resin (Table II). The test procedure modification applies to the change in leachant changeout schedule to allow all changeouts to be completed during the normal work week. The ANSI/ANS 16.1 test entails 11 leachant changes. The abbreviated version is based on the first seven leachant changeouts and was accomplished in seven days. The leachant used for these tests is ultra-pure water of resistivity 18.1 Mohm· cm. At this purity level, water is very aggressive and can extract ions even from metals, causing pitting corrosion.
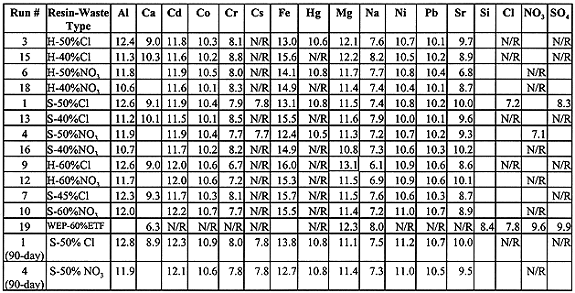
The Leachability Index (LI), a parameter defined for each leached constituent, is limited to the waste form composition for which it was determined. This index is applicable only when the predominant rate determining process during leaching is bulk diffusion. It is proportional to the logarithm of the inverse of the effective diffusivity of the contaminant (2). The diffusivity in turn is proportional to the square of the contaminant concentration in the leachates. The LI should be greater than 6.0 (4).
The LI is calculated for each leaching interval, then averaged over the seven intervals. Table III gives this average LI for the various species. Items left blank imply that those analytes were not present in significant amounts in the test specimen and N/R entries indicate that the leachate was not analyzed for this analyte. For Cd, Co, Fe, Ni, and Pb, the contaminant levels were below the detection capability of ICP-AES (Inductively Coupled Plasma - Atomic Emission Spectroscopy). For Run 4 which used the nitrate waste there were no chloride or sulfate anions present. In all cases where the analyte was below detection levels in the leachate, the detection limit value was conservatively used for LI calculations. For those contaminants, the true LI will be higher than the values presented.
All LI values are above the minimum requirement of 6.0 (4) with no significant correlation between the LI values and waste type, loading, or resin used (Table III). The vinyl ester and orthophthalic resin exhibited similar performance. Despite very small differences in LI values for Ca, Cr, and Fe, there is an expected correlation that higher waste loadings lowered the LI. For Cd, Co, Mg, Na, Pb, and Sr, there is no correlation, while for Al and Ni there appears to be an anomaly, i.e. lower LI at lower waste loading. The LI of all specimens being above the minimum LI of 6.0 clearly indicates that the toxic metals and radionuclide surrogates (cesium, strontium and cobalt) are well immobilized.
The increase in the number of samples being static-leach tested resulted in increased analytical costs. To stay within budget, the range of analyses was limited, e.g., out of 98 leachant samples, only 28 samples were subjected to mercury analysis by the cold vapor AA (Atomic Absorption) technique and cesium analysis by ICP-MS (Mass Spectroscopy). The test was abbreviated for all but two specimens (runs 1 and 4) that were extended to 90 days. For these runs Table III indicates that the LI for most analytes improved during the 83 days beyond the initial seven-day period.
Table III. Average Leach Indices
Toxicity Characteristic Leaching Procedure
The TCLP test was performed on six samples. These runs comprised the three resins at 50 percent loading of chloride and nitrate wastes. Results of leachate analysis are presented in Table IV, which includes the maximum allowable contaminant concentration (3). The lead, chromium, and TCE concentrations were within permitted levels in all samples while mercury concentration in Run 3 was borderline. No leachate met the criteria for cadmium.
The concentration of Cd is lower than Cr in the leachate. However, the TCLP limit for Cd (1 mg/L) being lower than that for Cr (5 mg/L) became a reason for failure in Cd. The contaminant compounds were added as oxides that have low solubility in water. Interactions with other waste constituents would allow some metals to form soluble compounds easily, e.g. cadmium chloride, and would enhance solubility based leaching. Another explanation for differing leaching rates is based on atomic/ionic sizes. Larger atoms (Pb>Hg>Cd>Cr) are expected to have lower diffusion-based leaching. The leachate concentrations in Table IV generally conform to this expectation.
It is suspected that the attritional TCLP specimen size-reduction method had a deleterious effect on the results. To confirm this hypothesis at the least cost, the TCLP test was repeated on one batch only using a different sample preparation technique.
Table IV. TCLP Results
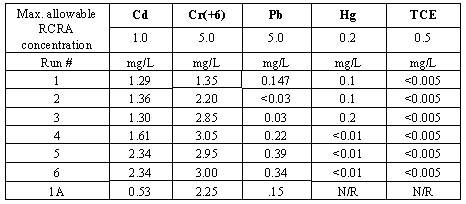
The sample preparation method was simplified and the TCLP test repeated for the run with orthophthalic resin and 50 wt.% chloride waste (Run 1). A 200-mL resin-waste mixture was prepared and poured into a special mold with cavities to yield 8 mm diameter pellets that were sized for direct use in the TCLP test. The result of this test is shown in Table IV as Run 1A. All measured contaminants, including cadmium, were below allowable limits. Mercury and TCE analyses in this leachate were not performed due to their added cost. This test proves that to evaluate true leaching characteristics in the waste form, the TCLP sample preparation method is crucial. Attritional sample preparation techniques compromise the encapsulating binder layer and mask the waste form's inherent encapsulating properties.
Water Immersion Test
All samples undergoing the water immersion test (4) maintained their integrity and were tested for compressive strength at the end of 90 days. As shown in Table V, chloride waste samples with the two types of resin easily met the requirement of maintaining at least 75 percent of their original strength and passed the 90-day water immersion test (4).
Table V. Post 90-day Water Immersion Compressive Strength
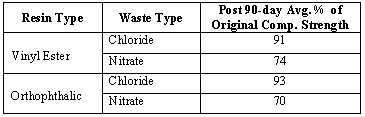
For the nitrate waste samples, the test needed to be extended to 120, 150, and 180 days to see if the strength leveled off and was above 3.45 Mpa (500 psi). However, this could not be accomplished due to an insufficient number of samples.
SUMMARY AND CONCLUSIONS
The tests proceeded at the anticipated pace without any unusual difficulties. Initial batches allowed determination of waste loadings and initiator proportions to be used. The factorial experiment design covered three classes of resin and two waste types at two loading levels. All test methods were observed and approved by a quality assurance engineer. A potential problem with high peak temperatures was resolved. The test findings are summarized below:
The objective of the surrogate tests has been achieved by demonstrating a high loading of generalized mixed wastes in polyester resins as a method of stabilization of salt wastes. The scope of work did not include optimization of waste, resin, or initiator proportions. Such optimization should be conducted with stream-specific compositions and physical properties of actual wastes.
FOOTNOTE
* AropolÔ is a trademark and HetronÒ is a registered trademark of Ashland Chemical Co., Columbus, Ohio.
REFERENCES
1. "Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens," ASTM C 39 - 93a, American Society for Testing and Materials, West Conshohocken, PA (1993).
2. "Measurement of the Leachability of Solidified Low-Level Radioactive Wastes by a Short-Term Test Procedure," ANSI/ANS - 16.1, American Nuclear Society, La Grange Park, IL (1986).
3. "Toxicity Characteristic," 40 CFR, Part 261 Ch. 1, '261.24, U.S. Environmental Protection Agency, Washington, D.C., (July 1, 1996).
4. "Technical Position on Waste Form," Technical Branch, Division of Low-level Waste Management and Decommissioning, U.S. Nuclear Regulatory Commission, Washington, D.C. (1991).
5. "HetronÒ and AropolÔ Resin Selection Guide," Ashland Chemical Company, Columbus, OH (1995).
6. "Immobilization of Low and Intermediate Level Radioactive Wastes with Polymers," Technical Reports Series No. 289, International Atomic Energy Agency, Vienna (1988).
7. WU, WEN-PAO, "Encapsulation of Hazardous Wastes in a Polyester Matrix," Master of Science Thesis, Department of Materials Science and Engineering, Washington State University, Pullman, WA (1978).
8. "Kinetic Mixer Demonstration," entry (pp. 42) in Progress Report, U.S. Department Of Energy, Mixed Waste Focus Area, Idaho Field Office (October/November 1997).