
TREATMENT AND CONDITIONING OF RADIOACTIVE LIQUID
WASTE BY ALGERIAN BENTONITE
M.S. Hamlat, B.Baggoura, K.Mansouri, S. Chelbani and N.Rabia
Centre de Radioprotection et Sûreté, 2, Bd Frantz-Fanon B.P. 399 Alger-Gare Algeria
ABSTRACT
This paper describes the studies carried out in the waste radioactive management laboratory, of the Centre de Radioprotection et Sûreté (CRS) to evaluate the Algerian bentonite properties, as sorbent and matrix for treatment and immobilization of low level radioactive liquid waste. The main composition of the bentonite under study are (SiO2/AL2O3) minerals. The fixation of radionuclides from low level radioactive liquid waste, such as 137Cs and 238U on natural bentonite, were investigated using static method for different liquid-solid systems conditions. Results showed that the bentonite has relatively a high degree of fixation for 137Cs. On the other hand, leaching phenomena onto bentonite matrix contaminated with 137Cs, was quantified using soxhlet technique for different experimental conditions. Leach rates obtained being very low, it is concluded that the bentonite can be eligible for safe conditioning of low level radioactive liquid waste.
INTRODUCTION
A management option of radioactive liquid waste produced in the nuclear industry and in radionuclide applications is its of immobilization and confinement in a repository.
To achieve this, the sorption that is the property of some materials (bentonite) to retain specific radionuclides is uti lized.
The ability of any particular waste /matrix combination to resist release of radionuclides into water is assessed using some kind of leach test carried out in the laboratory on miniature waste forms. To meet this end, batch and soxhlet tests have been carried out in laboratory using a wide variety conditions of sorption and leaching procedures.
This paper will consider the treatment-immobilization of the(137Cs/(cement/bentonite)) waste form combination characterized by two parameters: one describing the retention capacity using the batch technique and the other describing the leaching capacity using the soxhlet technique.
EXPERIMENTAL
Sampling
Natural bentonite from Maghnia (Western Algeria) was used as an adsorbent for these series of experiments (98 % of montmorillonite). Some physical and chemical compositions of the bentonite given by the supplier ‘ENOF’ (Entreprise Nationale des Substances Utiles et des produits non-ferreux, Algiers, Algeria) are reported in Table I.The chemical composition of ground water samples determined using classical methods is showed in Table II.
Treatment Experiment
The aim of the treatment is to test bentonite capacity to retain radioactive liquid waste. To achieve this, the sorption process is performed applying the batch technique to evaluate bentonite retaining efficiency using the distribution coefficient (Kd values). Kd is defined as the ratio of the activity per gram of solid to the activity per ml of the equilibrium solution [1]. For the 137Cs sorption experiment, 1 ± 0.1g of bentonite was dispersed into 20 ml of water containing 27.93 Bq of tracer and 1.12 10-2 M of carrier. The samples were incubated in 50 ml centrifuge tube during 1 hour at 250 rpm. After the equilibrium period the solid and liquid phases were separated by centrifugation (2000 rpm, based on the sedimentation theory and Stockes law [ 2 ]. The Kd values were calculated from the activity (Gamma counting) in the solution before and after incubation . The Kd evaluation and sorption isotherms were determined using the same experiment. The experiments were carried out in triplicates.
Leaching Experiment
The aim of the conditioning is to immobilize the treated radioactive waste. To achieve this, the conditioning process using cement/bentonite matrix was carried out. The quality evaluation of the conditioned products, was realised using the leach test. Waste form used was a mixture of ordinary portland cement and natural bentonite in 1/2 and 1/3 ratios. The 1/3 ratio is used according to litterature inherent concrete grades [ 3 ]. However, the ½ ratio was to evaluate and compare its results to that of the 1/3 ratio. The radionuclide 137Cs (0.3 mL of 6.10-3 Ci.mL-1) was dissolved into the water to mix with cement/bentonite and no inactive carriers were added. The 137Cs provides a broad interaction cross section in that this radionuclide is soluble in the the aqueous phase but is not taken into solid solution. All samples were obtained in the form of cylinders of approximately 25 mm in diameter, which after casting and vibro-compacting, were cured ( 2 days at 25°C) for testing the matrix breaking resistance in the leach test conditions. The leach test used will be refered to as low flow "ISO" soxhlet.The leachante used was at 25°C. In the soxhlet test [ 4 ], the specimen was exposed to a continuous stream of fresh aquifer water in a chamber which empties by syphonic action, when filled up. The emptying frequency was approximately once per hour and the effective flow rate approximately 0.22 L per hour. After the passage of convenient period of time, the leachate was analyzed and replaced with fresh leachant. The radiochemical analysis of the different solutions was carried out using Gamma counting.
The same experiment set up was used for different bentonite size (<f £ 335 m m; 355<f £ 500 m m and 500<f £ 900 m m) keeping the other parameters unchanged.
RESULTS AND DISCUSSION
Sorption Experiment
Bentonite and water Characterization: Table I shows the bentonite characterization results given by 'ENOF'. The high SiO2 /Al2O3 oxide contents is typical of the Maghnia bentonite. Other oxides of minor importance are also present in low percentages. Bentonite pH value (8.5) was also typical. The ground water chemistry was determined using classical methods. Table II shows that the water has an Na-HCO3 to more Na-Mg-Ca-K-Cl -SO4 composition. The existing monovalent cations such as K+, Na+ and NH4+ can inhibit more than those bivalent (Ca++ and Mg++) the 137Cs sorption. According to [5], 137Cs sorption is more importante for the fine particles with a developed specific area and a high retention capacity of mineral such as SiO2 /Al2O3. It was reported that [6] SiO2 /Al2O3 have a specific surface area of 50 and 100 m2.g-1, respectively. Thus, it results that the bentonite characterization with a high SiO2 /Al2O3 oxide contents and 98 % of montmorillonite can play a relevant role in sorption capacity.
Table I. Bentonite Chemical Composition

Table II. Water Chemical Composition
Effect of time: In order to determine the equilibrium time, five agitation times were considered: 10, 20, 40, 60, 120 mn. The other operating conditions were kept unchanged. Table III, shows that sorption growth is obtained at 15 mn with the establishment of an apparent equilibrium between the two phases at approximately one hour. Initially, the sorption rate is rapid. This is probably due to the availability of a great number of vacancies/sites. A plateau region is then reached, since fewer vacancies/sites are left as the reaction proceeds, and finally an equilibrium state is obtained between the two phases in the sorbate.
Table III. Distribution Coefficients of 137Cs in Various Conditions
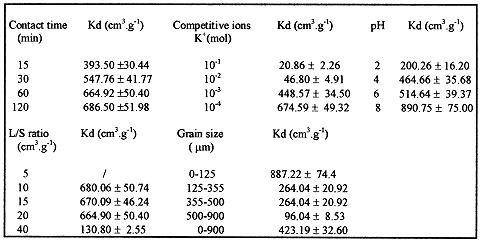
Effect of liquid-solid ratio (v/m): The operating liquid-solid ratios used were: 20/0.5; 20/1; 20/1.5; 20/2. Table III shows that the Kd is improved at the least liquid-solid ratio. This is attributed to an increasing surface area for higher solid-liquid ratios. A small quantity of bentonite is more quickly saturated than a larger one when no breaking occurs during the process [7].
Effect of pH: The pH was adjusted with either a reagent grade HCl or NaOH, the operating pH values were: 2;4;6;8; and 10. The pH effect on sorption can be, at least in a major part, predicted from the e =f(pH) variation (e -potential is the essential parameter to be considered since it reflects both stability and surface charge of the oxides) [8]. It was reported that [9], as pH increases, the charge of oxides becomes less and less positive (Al2O3 /TiO2) or more and more negative (SiO2), resulting in an increasing sorption ratio for 137Cs ( the case of SiO2). Moreover, 137Cs is more easily sorbed on SiO2 than on TiO2 , under the same experimental conditions. Table III shows that Kd values increase when the pH increases. However, there is an exception for Cs/ SiO2 system, because, N.Hakem [10] has shown that the sorption as function of pH presents 3 different steps: constancy, increasing and then decreasing. Thus, the sorption behavior of 137Cs on bentonite, could probably be due to the formation of specific species such as: SOH + Cs+— SOCs + H+, over the whole pH range (2 to 10).
Effect of particle size: The operating grain sizes used were : 0-125 µm; 125-350µm; 350-500µm; 500-900µm. Table III shows that the distribution coefficient is strongly higher when the particle size decreases. This is due to the fact that the surface area increases with decreasing particle size.
Effect of competitive ion: The operating competitive ion used was : K+ concentration (3, 10, 15, 20 mg.L-1 ). The environmental water used does not contain a significant amount of dissolved cations and anions of varying concentration. Table III shows that the distribution coefficient decreases when the K+ concentration increases. This may be due to the fact that the sorption is seriously inhibited by K+ , having steric favorableness for edge fixation sites.
Equilibrium isotherm: The equilibrium adsorption isotherms are of fundamental importance in determining the sorption capacity onto soil. In Order to correlate the adsorption isotherms, data were fitted applying the models of Langmuir and Freundlich discussed below [11]
Langmuir model: The Langmuir theory was first used to describe the adsorption of gaze molecules onto metal surfaces [12]. However, this model has found successful applications in many other sorption processes. According to be basic assumptions of the Langmuir model, the amount of 137Cs adsorbed can be expressed by:
Qe= X/m = Qo.b.C / 1 + b.C |
(1) |
A linear form of this equation is:
| C/Qe= 1/Qo.b+ C/Qo | (2) |
Where:
b= Langmuir isotherm constant (L.Bq-1);
C= equilibrium liquid phase 137Cs concentration (Bq.L-1);
X= mass of 137Cs adsorbed onto bentonite ( Bq);
m= mass of bentonite (g);
Qo= Langmuir monomolecular layer (Bq.g-1);
Qe= equilibrium solid-liquid 137Cs concentration (Bq.g-1).
Figure 1 illustrates the Langmuir analysis of the isotherm data for 137Cs. The values of Qo and n are calculated by the least-squares method. Results are presented in Table IV. In this particular case, the shape of the isotherm remains identifiable only at lowe concentrations. Consequently, the Langmuir approach is not satisfactory for this type of studies.
Table IV. Langmuir and Freundlich Isotherm Constants

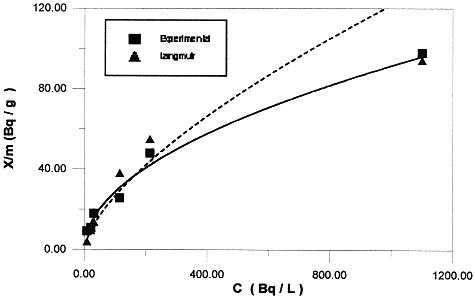
Fig.1. Application of the Langmuir equation to the sorption of Ceasium-137 into bentonite.
Freundlich model: The Freundlich model stipulates that the ratio of solute adsorbed to the solute concentration is a function of the solution. The empirical model has been shown to be consistent with an experimental distribution of active centers characteristic of heterogeneous surfaces. The amount of solute adsorbed X/m, is related to the concentration of solute in the solution, C, as indicated by the following relation:
X/m = K.Cn |
(3) |
This expression can be linearized to give:
logX/m = logK + n logC |
(4) |
Where:
k = Freundlich isotherm constant (L. g-1)
n = Freundlich isotherm constant.
Both K and n constants, this is indicative of the extent of adsorption and the degree of non-linearity between solution and concentration, respectively. Figure 2 illustrates the Freundlich analysis of the isotherm data for 137Cs. The values of K and n are calculated by the least squares method. Results are presented in Table IV. For this case, the shape of the isotherm remain unchanged over the whole concentration range studied. The 137Cs isotherm can be described by the Freundlich equation. The best fit line of logX = log 0.08 + 0.53logC has a linear correlation coefficient of 0.99.
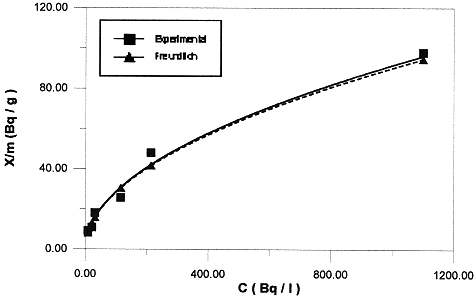
Fig. 2. Application of the Frendlich equation to the sorption of Ceasium-137 into bentonite.
Leaching Experiment
The results are expressed in terms of leach rate, Lr, given by the following expression [13]:
Lr = A . M/ Ao.S.t |
(5) |
where A(Bq) is the activity leached into the liquid up to the time t, Ao (Bq) the total activity of the solid sample at t=0, M (g) the mass of the solid sample (including internal porosity), S its geometrical surface area and Lr, the leach rate of sample (quantity of radionuclide leached by time per surface area). In this case, only the activity which had emerged from the leaching cell has been included in calculating Lr. By applying the error theory [14] to this case the formula for the standard deviation of the Lr value was deduced, as a result of the propagation of the introduced errors. The leached activity and leach rate results for 137Cs for different conditions are shown in Fig. 3 and 4, respectively.
Leached activity: Determination of the released activity in a large range of grain sizes (350 to 900 m m) were carried out for 1/2 and 1/3 cement/bentonite ratios. Figure 3, shows the released activity increases and decreases for both ratios with the grain size range used. This may be due to the fact that the leachant extracts the activity deposited on the external sites. After this, the release decreases giving a smaller value from the internal sites due to the limited penetration. However, the highest released activity results from the 1/3 ratio sample with an increasing grain size.
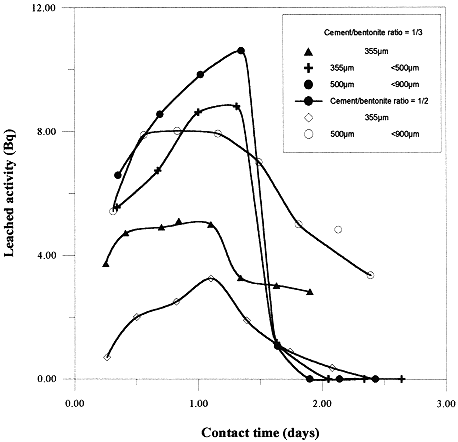
Fig. 3. Leached activity of ceasium-137 as function of time.
Leach rate: In order to determine the leach rate, different grain size tests as function of time were carried out. Grain size influence on leaching was determined using the 1/2 and 1/3 ratios. The results are presented on Fig. 4, where it can be observed that the leaching decreases with decreasing grain size. This is attributed to the increasing surface area of bentonite. Furthermore, the leach rate of both 1/2 and 1/3 ratios samples over the grain size range remain unchanged , except for 355 µm where, Lr (1/2) is greater than Lr(1/3).
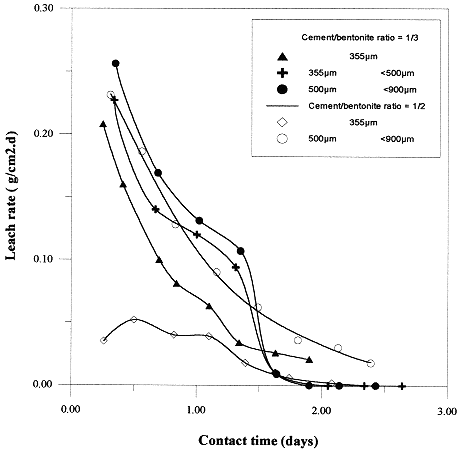
Fig. 4. Leach rate of ceasium-137 as function of time.
CONCLUSION
Batch method is mostly effective for studying radionuclides behavior without mobility matrix media. On the other hand, the dynamic method is used to simulate migration of radionuclides through matrix media. Furthermore, the choice of the 'ISO Soxhlet' is of its simple laboratory use conditions.
A study of sorption and leaching on natural bentonite was undertaken using batch and soxhlet tests under a variety of conditions. The results indicate that the range of Kd values is 20 to 900 cm3.g-1 . The Algerian bentonite has a good selectivity sorption for 137Cs, due to the high specific surface area of the fine particles of SiO2 /Al2O3 oxides. The equilibrium adsorption isotherms of 137Cs onto bentonite seems to be well described by the Freundlich model.
The leaching capacity is characterized by the leach rate. The soxhlet test was selected for its simplicity. The leach rates obtained (10-3 to 10-1 g/cm2 d) were used for the analysis of the 137Cs release capacity. Hence, according to the obtained results, a cement/bentonite used in 1/2 ratio, is acceptable for the safe conditioning of radioactive liquid waste. However, for relating the results of both methods, more studies, such as mathematical simulation of leach tests to be carried out in the future.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Yahiaoui 'ENOF' (Entreprise Nationale des Substances Utiles et des produits non-ferreux, Algiers, Algeria) for his kind help in the sampling phase of this work.
REFERENCES