TREATMENT OF NUCLEAR WASTE EFFLUENTS BY HIGHLY
SELECTIVE INORGANIC ION EXCHANGE MEDIAS-
EXPERIENCE GAINED AND NEW DEVELOPMENTS
R. Harjula, J. Lehto and L. Brodkin
Laboratory of Radiochemistry
Department of Chemistry
PO Box 55, 00014 HELSINKI UNIVERSITY, Finland
E. Tusa and J. Rautakallio
Selion OY
Rajatorpantie 8
01600 Vantaa, Finland
ABSTRACT
The increasing cost of final disposal is making nuclear sites to look for new ways to decrease their radwaste volumes. One way to achieve this is to use highly selective ion exchange media, e.g., to replace evaporators or to replace conventional resins in demineralizer systems.
The highly efficient CsTreat® ion exchange media, manufactured by Selion OY, Finland, was first taken into use at Loviisa NPP (PWR, VVER-440) in 1991 for the treatment of high-salt (240 g/L) evaporator concentrates. Very large volume reduction and decontamination factors have been obtained for these liquids, which has brought considerable savings in the waste treatment. Since 1991, CsTreat® media has been chosen in several other NPP's and nuclear sites to process both high-salt and low-salt cesium-bearing waste waters. Another Selion OY media, SrTreat® was first taken into large-scale use at Murmansk, Russia, in 1996 to purify wastewaters originating from nuclear icebreakers.
This paper will describe applications of CsTreat® and SrTreat® that have been commissioned or will be commissioned in the near future in NPP's (Loviisa NPP, Finland; Olkiluoto NPP, Finland; Callaway NPP, USA and Paks NPP, Hungary) and other nuclear waste projects (Paldiskij, Estonia; Murmansk, Russia; JAERI, Japan).
In addition, a new selective ion exchange media, CoTreat, has just been developed. CoTreat is highly selective for radiocobalt and other activated corrosion product nuclides (e.g. 54Mn, 59Fe, 63Ni, 65Zn), which are important in the NPP field and for TRU nuclides (e.g. 236Pu). Laboratory scale test program is underway to evaluate the performance of CoTreat in various types of waste solutions. In the first phase, which is soon completed, various simulated solutions were used. Test results indicate that very high processing capacities are obtainable for CoTreat. Selected results on these tests will be presented. In the second phase of tests, which will commence in February 1998, actual waste solution from several utility sites will be used.
THE SELECTIVE ION EXCHANGE MEDIAS
CsTreat® is an all-inorganic, transition metal hexacyanoferrate based ion exchange media, which is extremely selective for cesium. The selectivity of CsTreat® is higher than that of any other commercial Cs-selective media1. SrTreat® is an all-inorganic, titanium oxide based media, which is highly selective for strontium and e.g. for plutonium. CoTreat is a new titanium-based material, which is selective for cobalt (e.g. 58Co), manganese (e.g. 59Mn), iron (e.g. 59Fe), zinc (e.g. 65Zn) and plutonium (e.g. 240 Pu). All these medias are produced both in granular form (column operation) and in powder form (pre-coat application).?Because of high selectivity, the materials have a very high processing capacity, even in cases that the water has high concentrations of non-radioactive salts. High capacity means that there will be a very low volume of final waste to be disposed of.
EXPERIENCE GAINED
Sites Using Selion Medias
The sites using Selion ion exchange medias include:
Loviisa NPP (PWR), Finland
CsTreat® media has been in use since 1991 for the treatment of high salt (240 g/L) evaporator concentrates2. Average processing capacity of the media has been 66,000 gal/ft3 and the decontamination factor (DF) for 137Cs has been above 1,000. Several million USD have been saved in the waste treatment costs after CsTreat® was installed.
Olkiluoto NPP (BWR), Finland
CsTreat® was taken into use in April 1997 to treat low-salt floor drain wastewaters. In the first stage, 240 m3 (63,000 gal) of water in storage tanks was purified using a single 12-L (0.43 ft3) column of CsTreat® with no sign of bed exhaustion. The DF obtained for 137Cs was dependable on the flow rate3. Purification campaign was first started at a flow rate of 20 BV/h and DF’s above 1,000 were obtained. Due to time constraints, flow rate was stepwise increased to 50 BV/h, which resulted in a gradual decrease of DF. At 50 BV/h, the DF stabilized to a value of about 100.
Callaway NPP (PWR), USA
CsTreat® media was installed in the filter/demineralizer system to replace evaporator at Callaway NPP in July, 1996 3. The CsTreat® bed size is 9 ft3. Since the start-up, some 500,000 gal of low-salt water has been treated with the same bed. The activity of 137Cs in the demineralizer effluent has been non-detectable after efficient filters were installed to remove particle-bound cesium from the influent. The use of CsTreat® in the demineralizer system has reduced the liquid radwaste processing costs by 50 %4.
Paldiskij, Estonia
In 1996, a total of 200,000 gal of various waste waters accumulated in the operation of ex-Soviet naval training reactors were purified using a single 0.43 ft3 CsTreat® column. No sign of bed exhaustion was observed when the treatment campaign was completed, which means that the processing capacity of CsTreat® exceeded 465,000 gal/ft3.
Murmansk, Russia
In 1996-97, a transportable NURES unit utilizing CsTreat® and SrTreat® beds (0.43 ft3), prefilters and a carbon filter was used to treat radioactive wastewaters accumulated from nuclear-powered icebreakers5. Maximum DF's for 137Cs and 90Sr have been 1,000 and 5,000, respectively. The NURES system appeared to be efficient for the removal of other radionuclides, e.g. 60Co and 125Sb, too.
JAERI, Japan
CsTreat® and SrTreat® were taken into use at Japan Atomic Energy Research Institute (JAERI) Tokai site for the removal of 137Cs and 90Sr from alkaline reprocessing waste effluent in the summer of 1997.
Sites That Have Chosen Selion Media
Paks NPP, Hungary
A new liquid waste treatment system, including utilization of CsTreat® , is in detailed design phase at Paks NPP, Hungary (PWR, VVER-440) and the operation, which may start in 1998, will be very similar to that at Loviisa NPP. Over 500,000 gallons of evaporator concentrates and other liquids have been accumulated in the storage tanks at Paks NPP for treatment.
TESTING PROGRAM FOR CoTREAT
Phase 1 Experimental
In the first phase CoTreat was tested using three types simulated solutions: a NPP floor drain water (FDTW), sea water and an evaporator concentrate, representing low-, medium- and high salt liquids (Table I). Solutions were tagged with key corrosion product nuclides ( 59Mn, 57Co, 59Fe, 63Ni, 65Zn) and a TRU nuclide (236Pu).
Table I. Composition of Simulated Waste Solutions

The tests comprised of static batch experiments and column experiments. In the batch experiments the distribution coefficient (kd) of different radionuclides was determined as a function of pH in the waste simulants. CoTreat was pre-equilibrated with non-tagged solutions prior to kd determination so that there would be no change in the solution of ion exchanger composition. The kd- results are thus representing the same equilibria that would be attained in column operation. Under these circumstances, the kd-value also gives the maximum theoretical column capacity, in terms of solution volume (L) that can be purified per unit mass (kg) of exchanger1. The kd-determinations were carried out by equilibrating CoTreat samples in tagged solutions (20-mL solution/0.05 g exchanger) for two weeks. After equilibration, the solution was centrifuged, filtered and the radionuclide concentrations were analyzed. The values of kd were calculated using a formula:
|
(1) |
Where ![]() and A are the equilibrium
activity concentrations of the radionuclide in the exchanger and in the solution,
respectively, A0 is the activity concentration initially, V is the solution
volume (20 mL) and m is the exchanger mass (0.5 g).
and A are the equilibrium
activity concentrations of the radionuclide in the exchanger and in the solution,
respectively, A0 is the activity concentration initially, V is the solution
volume (20 mL) and m is the exchanger mass (0.5 g).
Column experiments were carried out using small lab-scale columns (bed volume 0.5-1 mL.) The flow rate was 10-20 bed volumes/hour and exchanger grain size 0.85-0.25 mm. Column effluent was collected as fractions, which were analysed for the activity concentration of different radionuclides.
Experiments with FDTW were conducted at four different pH-values (2,5,7,11) to see the effect of pH on the column performance. The pH of the feed solution was adjusted using HNO3 or NaOH. Relatively low throughput, about 2000 bed volumes, was used in the FDTW experiments. The pH of sea water simulant was lower (4.6) than what one would expect in real sea water because some the added radionuclide solutions were highly acidic and thus decreased the pH.
Phase 1 results
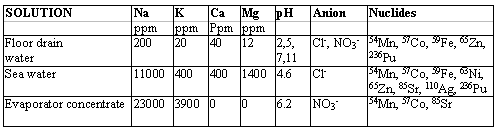
Distribution coefficients vs. pH.
The general trend for all the radionuclides investigated was that the distribution coefficients increased with increasing pH in the acidic range, leveling-off at neutral pH's. In alkaline region, kd started to decrease again (see example in Fig. 1). In the acidic range, this behavior is typical of an weak-acid type exchanger: at low pH, the exchanger is mostly in non-dissociated hydronium form and metal ion uptake (and kd) are low. The degree of dissociation increases as pH increases with simultaneous increase in metal ion uptake. In alkaline region, the hydrolysis of the metal cations increases with pH and an increasing proportion of these are present as neutral and anionic species. These species are not cation exchangeable, so a decrease in kd is observed. In FDTW and evaporator concentrate simulants, the maximum kd-values for 54Mn, 57Co, 63Ni, 65Zn and 236Pu (FDTW only) were in the range of 100,000 – 1,000,000 L/kg indicating that column capacities in excess of 100,000 L/kg (500,000 gal/ft3) could be achievable for these nuclides with CoTreat media. In simulated seawater, the maximum kd for 57Co was somewhat lower (about 50,000 mL/g) and for 63Ni very much lower (about 2,000 mL/g). This indicates that Ca and Mg, which were abundant in the water, have a decreasing effect on the uptake of these nuclides.
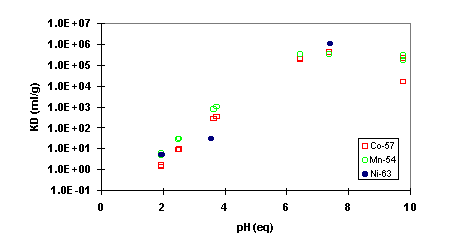
Figure 1. Distribution coefficient kd for 54Mn, 57Co and 63Ni in simulated evaporator concentrate (for composition, see Table I) as a function of pH.
Column experiments
Floor Drain Tank Water: In the FDTW, the highest decontamination factors (DF) were obtained when the feed pH was adjusted to 5 (Fig. 2). The DF for 57Co was well above 1000 throughout the column run. The activity concentration of 65Zn was below detection limit in most analyzed fractions. Calculated from the minimum detectable activity the DF was at least 1000. The situation was similar for 59Fe, but because the activity in the feed was low, calculation from the minimum detectable activity yields only that DF > 50 (in reality, DF may have been much higher than this figure). The DF for 54Mn was around 500 and that for 236Pu 30-50. At feed pH = 7, the DF's were considerably lower (e.g. 57Co DF » 100, 54Mn DF » 100). No sign of column exhaustion was observed when the experiment was terminated at about 2000 BV. This was expected from the kd-determinations, which suggested column capacities in excess of 100,000 mL/g (» 100,000 BV) in the neutral pH range. At pH = 2, all DF's were very low and the column was exhausted already when about 500 bed volumes of solution had been treated.
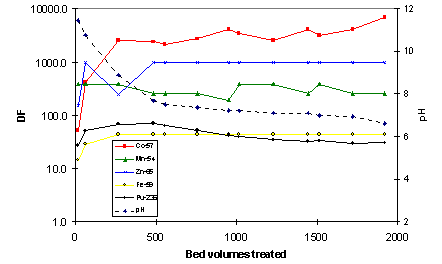
Figure 2. Decontamination factor DF for various radionuclides in simulated Floor Drain Tank Water as a function of bed volumes (BV) treated. CoTreat bed 0.5 mL, flow rate 10 mL/h. Activity concentrations of 59Fe and 65Zn were below the detection limit in most analyzed samples and for those the DF's have been calculated from the minimum detectable activity.
Evaporator concentrate: No sign of column exhaustion was observed for the CoTreat column in the high-salt evaporator concentrate, even though almost 20,000 BV of solution had been treated at the termination of the test (Fig. 3). The DF for 57Co and 54Mn remained well above 1000 throughout the test. These results are quite remarkable, considering the high salt content of the
Simulated seawater: In the simulated seawater the DF-levels for the radionuclides were mostly clearly lower than in the evaporator concentrate (Fig. 4). In addition, DF’s had a continuous downward trend. At 20,000 BV, CoTreat column still took up 59Fe and 65Zn with a reasonable DF, but the capacity was almost exhausted for 54Mn and 57Co. The capacity for 63Ni was exhausted at about 10,000 BV and for 85Sr and 110mAg already before 1000 BV (data for for 63Ni, 85Sr, 110mAg has been omitted in Fig. 4 for clarity). These lower capacities are most likely attributable to the presence of considerable amounts of Ca (400 ppm) and Mg (1400 ppm) in the solution.
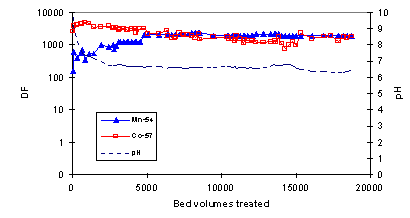
Figure 3. Decontamination factor DF for 57Co and 54Mn in simulated evaporator concentrate as a function of bed volumes (BV) treated. CoTreat bed 0.5 mL, flow rate 10 mL/h.
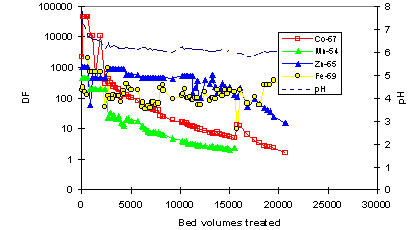
Figure 4. Decontamination factor DF for 65Zn, 59Fe, 57Co and 54Mn in simulated seawater as a function of bed volumes (BV) treated. CoTreat bed 0.5 mL, flow rate 10 mL/h. Data for for 63Ni, 85Sr, 110mAg has been omitted for clarity.
CONCLUSIONS
Experience gained in several sites shows that CsTreat® is a very efficient ion exchange media. An average processing capacity of 66,000 gal/cu.ft has been obtained at Loviisa NPP for a high salt effluent (240 g/L) even in the presence of potassium, which is known to decrease cesium uptake strongly. The processing capacity increases when the salt concentration decreases. So far, no exhaustion of CsTreat® bed has been observed in applications involving low-salt effluents, and the highest observed throughput has been 465,000 gal/cu.ft with no sign of bed exhaustion. Laboratory-scale column tests have been conducted upto 1,500,000 gal/cu.ft with no cesium breakthrough. The use of CsTreat® media has brought considerable savings in the liquid waste treatment for the utilities. At Loviisa NPP, several million USD has been saved. At Callaway NPP, liquid waste treatment costs have been reduced by 50 %. SrTreat® media has proven to be very efficient for the removal of 90Sr from low-salt and high-salt liquids.
As to parameters that impact operational conditions the effect of flow rate on DF often needs special consideration. Due to high selectivity, very small beds of selective media are needed in most applications if only processing capacity, in terms of L/kg (or gal/ft3) is considered. However, to obtain high DF’s, the flow rate should be relatively low, in the range of 10-30 BV/h. For a small bed this means that the throughput rate, in terms of L/min (or gal/min), is low. In most applications the throughput rate must be high which means that the bed size must be made much larger than what would be required for a few years’ operating capacity. Large beds of selective media may have a projected operating life of tens of years based on their ion exchange capacity. The challenge then is to keep the bed operable over long periods of time, e.g. to avoid fouling of the bed by sludges or oils. Removal of sludges and particles from column feed solution is thus essential in the process, not only to protect the bed but also to remove radionuclides that are associated with suspended solid material in the solution. Operating experience has e.g. showed that even in the case of 137Cs, considerable proportion of total Cs-activity may be bound by small particles. If these particles are not removed, the overall DF may be relatively low, since the ion exchange bed only takes up dissolved cesium ions. In order to avoid bed fouling by oils or other organic substances, upstream active carbon beds have been found effective.
Based on the laboratory tests with simulated waste solutions, the new CoTreat media is showing great promise for the removal activated corrosion product nuclides from both low-salt and high-salt waste liquids. Because it is not possible to simulate the chemical form of these nuclides as they actually exist in real waste liquids, it is important to verify these results using actual waste. A test program for this will be soon underway.
REFERENCES
BACK