SUBSURFACE BURIED WASTE CONTAINMENT SYSTEM
MATERIAL TESTING
Peter Shaw, Jerry Weidner and Anne Glenn
Idaho National Engineering Laboratory
Lockheed Martin Idaho Technology Co.
2525 N Fremont
Idaho Falls, Idaho 83415-3710
ABSTRACT
This report describes results from laboratory scale testing of modified latex cement materials for in situ underground horizontal barriers. The objective is to formulate materials that are pumpable, set rapidly, are strong, have low shrinkage, low heat of curing and are durable . Various formulations of Rapid Set cement, DOW latex polymer/super plasticizer and a course sand aggregate were tested. Twenty seven tests gave material formulations suitable for field proof of principal demonstration of a novel subsurface barrier placement device. Cement and ASTM-33 sand between a 0.5 and 0.33 ratio, a water to cement ratio of 0.4, a latex to cement ratio of 0.15 and 0.5% citric acid were optimum. That is 100 parts cement, 200-300 parts sand, 40 parts water 15 parts latex and 0.5 parts citric acid. The material was workable for 15 minutes, had an initial stiff set (unconfined compressive strength (UCS) of 20 psi [0.14mPa] ) in 30-60 minutes, an average UCS of 700 to 1000 psi (4.8-6.9 mPa) in one to two hours, an average 28 day UCS of 3000 to 4000 psi (20.8-27.6 mPa), a bulk density of 2 g/ml and a specific gravity of 2.4 g/ml. 70% of this UCS was retained after 28 day leaching in soil or calcium/sulfate/carbonate saturated ground water or DI water with a 1% weight loss. The material was successfully used in a field proof of principal test demonstrated in Spokane. Preliminary results from this test show a UCS of 5000 psi (34.7 mPa) and a bulk density of 2.2. Workability and set times were suitable for the machinery.
TECHNOLOGY DESCRIPTION AND BACKGROUND
This report describes the results of the laboratory test program to validate the application of a latex-modified cement formulation for use with the Buried Waste Containment System (BWCS) process proof-of-principle demonstration. The objective of the laboratory testing was to validate the barrier material mix formulation to be used with a field demonstration of the BWCS equipment. The suitability of the basic mix formula, supplied by the cement and latex vendors, was verified by laboratory testing at the Idaho National Engineering and Environmental Laboratory (INEEL).
The BWCS is a technology for the in situ fabrication of a trough-shaped waste containment barrier beneath a waste site. The dimensions of the planned demonstration trough are approximately 40 ft wide with a thickness of 1 ft at the bottom and 6 in. on the sides. The depth to the trough bottom will be 20 ft. The trough material is to provide an impermeable barrier to contaminant migration; therefore, the material must have virtually zero crack density at the field scale and a hydraulic conductivity of 1 x 10-7 cm/s1 or less as measured in the laboratory. The shape of the structure and the low crack density require the material to either have very small volume change during set and cure or be capable of some plastic deformation without cracking. The BWCS process and placement equipment also impose some constraints on the physical properties of the material and the setting process.
The requirement for the initial set time is to develop an unconfined compressive strength (UCS) of 20 psi within 15 to 30 minutes. The UCS (ASTM C 39)2 at set must exceed 20 psi (0.14mPa) to support the soil overburden during the grouting process. This number is a conservative estimate based on an assumed soil overburden density of 1.2 g/cm3 to 1.6 g/cm3. 3 The load produced by the overburden at a depth of 20 ft would then be about 14 psi. The phrase "initial set time" as used in this paper is that time at which the cement mixture achieves a UCS of 20 psi (0.14mPa). The maximum set temperature is to be less than 100° C and maximum slump (ASTM C 143) is to be 8 to 10 in. Aggregate particle size must be in accordance with ASTM C 33 for fine aggregate.
The barrier material will be applied to a waste site in an arid environment and should have chemical properties that are compatible with the soil and groundwater typical of arid climates. Therefore, the barrier material must be resistant to chemical attack from solutions saturated with calcite—CaCO3 (high carbonate) , gypsum—CaSO4·2H2O (high sulfate), typical of caliche deposits in arid region soils and solutions extracted from the waste site soil.
Rapid Set®1 cement-based mortar, modified with latex (i.e., styrene butadiene rubber) was selected as the most reasonable candidate material for the BWCS POP application. The styrene butadiene rubber (SBR) material is a commercially available commodity used in the construction industry and has also been tested for waste barrier applications.4 The latex provides low-permeability, high-adhesion resistance to chemical attack,5 as well as resilience and resistance to crack formation. Rapid Set cement, a proprietary material manufactured by the CTS Cement Manufacturing Company, was chosen because it is sulfate-resistant, has minimal shrinkage, a set time less than 30 minutes without the addition of special accelerators, and has been tested with the SBR admixture. Latex-modified cement was preferred for this application because it has workability properties similar to conventional cement, is hazard free and environmentally safe, is easily cleaned up with water, and is expected to have satisfactory long-term durability properties.4
Taken together, the properties of the latex-modified Rapid Set mortar indicate an adequate barrier material for use in arid environments at a cost lower than barrier materials requiring two components or hazardous constituents.
MATERIALS
Standard equipment was used for the laboratory test program. The materials used in the test program are described below.
Rapid Set cement is a hydraulic cement manufactured by the CTS Cement Manufacturing Company. It is used in industry for applications such as bridge overlays and airport runway repair where high early strength and very low shrinkage are required. It is resistant to sulfate attack. It has a specific gravity of 3.1. Rapid Set cement was used to prepare all the materials discussed in this paper.
The latex cement additive, Modifier A, was manufactured by the Dow Chemical Company. Modifier A is a white liquid emulsion of SBR and other materials and has about 48 wt% solids. It has a specific gravity of 1.04. The latex provides low-permeability, high-adhesion resistance to chemical attack and crack formation. Latex Modifier A was the latex used in all materials discussed in this paper.
The sand used in most laboratory mixtures met the specification for concrete sand, ASTM C-33. In some initial laboratory tests, mortar sand, meeting specification ASTM C-144, was also used. The size specification for both sand lots was verified by sieving. The specific gravity is 2.5. The concrete sand contained 4±1 wt% water; the mortar sand contained 2.5±1 wt% water and was determined by weight loss at 105° C.
Citric acid, used as a set retardant, was a reagent-grade, solid material supplied by Fisher Scientific. It has a specific gravity of 2.2. The citric acid was always mixed with the cement prior to the addition of the latex in order to prevent an undesirable reaction between the basic latex and the acid.
EXPERIMENTAL PROCEDURES
The objectives of the barrier material laboratory tests include the determination of temperatures during barrier material curing, mixture compositions so that set time was between 15 and 30 minutes and slump was between 8 and 10 in (20.3 cm and 25.4 cm)., viscosity of mixtures as a function of time, and aqueous dissolution and stability studies of barrier materials. Because of the fast reaction rates of the materials, special procedures had to be developed for some measurements.
Laboratory Barrier Material Preparation
The laboratory test materials were prepared according to the following procedures. The dry cement and citric acid were first mixed together followed by the sand, then latex, and finally water. The mixing order was used in order to achieve complete mixing of the materials in the shortest amount of time without neutralizing the citric acid with the latex. Note that citric acid is added to and reacts with the cement before the latex emulsion is added in order to minimize the acid-base reaction between citric acid and latex. Small samples, 1 kg or less, were mixed rapidly in plastic beakers by hand until homogenous. The larger samples were mixed in plastic buckets with a small shovel. A mechanical mixer was not used because of the possibility of introducing too much air into the mixture. Where possible, techniques for mixing followed ASTM C-305, "Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency."2 Components in small batches, 1 kg or less, were weighed to the nearest 10 mg. Larger mixtures were weighed on a floor balance to the nearest 5 g. Sixteen different formulations were used for laboratory testing with cement contents ranging from 18-59% , sand from 0-70% and water from 10-32 wt%.
After mixing, the test material was used for viscosity measurements or slump measurements, or was molded into cylinders. In many cases, the material used for viscosity or slump measurements was molded into test cylinders after the measurements were complete. Cylinder sizes (diameter versus length) included 0.5 x 1 in., 1 x 2 in., 2 x 4 in., and 3 x 6 in. A thin coat of silicon vacuum grease was used as a release agent on the walls of the plastic cylindrical molds. In general, the test specimens were removed from the molds when manually pressing on the barrier material no longer produced an indentation, usually within 30 minutes after sample preparation. In the case of the set time determinations, the test specimens were removed as soon as they appeared to be self supporting. Usually 10 to 24 cylinders were prepared from each batch of material.
Viscosity, Unconfined Compressive Strength, Slump, and Temperature Measurements
Viscosity Measurements.
The viscosity of the latex mortar material was measured as a function of time using a Brookfield Digital Viscometer, Model DVII+, equipped with a Brookfield Helipath Stand and a T-bar Spindle. Procedures followed the manufacturer’s instructions, which were based on ASTM methods. Viscosity measurements were started as soon as the sample was mixed and visually determined to be homogeneous, usually within 2 minutes of the addition of water and latex. Mixture viscosity measurements were made using 80 to 120 g (40 to 60 mL) of material in a 100-mL plastic beaker.
Timed data point collection was made using the Wingather software provided by the manufacturer. The time was measured starting at the instant of the addition of the latex/water to the mixture. Mixture temperature, viscosity, and spindle rotational speed were measured and electronically recorded at 2-second intervals. When viscosity measurements reached 500,000 to a maximum of 1,000,000 centipoise, viscosity measurements were stopped and the material was transferred into molds to produce tests specimens for UCS and other measurements. Because viscosity measurements required less than 100 g of material and were easily and quickly prepared, viscosity was routinely determined on all batches of material.
Unconfined Compressive Strength and Density.
UCS was used to define initial set time, i.e., the time required to support a load of 20 psi, and also to provide an easily measured parameter to use as a monitor of barrier material change with time. Density was measured as part of the material characterization process.
UCS was conducted according to ASTM C 39-96 and ASTM D-1633 except that 1 x 2-in. cylinders were sometimes used rather than the prescribed minimum 2 x 4-in cylinders. Previous testing at the INEEL indicated that measurement precision was unaffected by sample size for this type of sample. When necessary, 1 x 2-in. cylinders with broken or uneven ends were supported in plastic boats containing sand to compensate for the uneven surfaces.
Measurements were made with an ECS G-900 Versa-Loader equipped with a Soil Cement Test Digital Readout Set. The system has a 10,000-lb capacity. Data were recorded manually from the digital readout system.
The UCS was measured by slowly compressing the cylindrical samples at a rate of 0.05 in./min. Pounds force was read on the factory-calibrated digital strain gauge. Typically, the exerted force increased gradually to a maximum, then fell off rapidly when the sample cracked. Irregularities of the sample shape or the presence of voids often produced multiple peaks or a very broad peak. This generally resulted in a lower maximum reading.
Two to six samples were used for the initial set time measurements. The remaining samples were stored in small, sealed, plastic bags. Systematic UCS measurements were made in the first hour, the first day, and then at 28 days after mixture preparation on most mixtures and 2 months on many of them. UCS was measured 30 minutes after batch preparation to determine the initial set time. Density was measured according to ASTM C-642, "Porosity, Specific Gravity, and Air Voids."
Slump.
Slump was determined according to ASTM C-143 using Slump Test Set CT-225 supplied by ELE International.
Slump was determined only on selected batches because slump measurements required at least 8 kg of material. Viscosity was determined simultaneously with slump, but using a separate sample from the same batch. Each slump test required about 3 to 5 minutes to perform and was repeated for a given batch of material until the slump measurement was less than 8 in., corresponding to a viscosity exceeding 500,000 centipoise. Each slump test was recorded manually as a function of time. Viscosity measurements were simultaneously recorded electronically.
Temperature Measurement.
Temperature was determined during viscosity tests using the temperature probe attached to the viscometer and was recorded electronically using the Wingather software. Mixture temperature during the setting and curing process was determined using an ethanol thermometer imbedded in the center of a 3 x 6-in. cylinder filled with uncured barrier material. Temperature measurements were manually recorded as a function of time until the temperature peaked and began to drop.
Aqueous Dissolution and Stability
The barrier material will be applied to a waste site in an arid environment and therefore should have chemical properties that are compatible with the soil and groundwater typical of arid environments. The following experiments provide information about the behavior of the barrier materials in different aqueous environments.
Laboratory-molded 1 x 2-in. cylinders were tested in three different aqueous solutions. One solution was saturated with respect to calcium carbonate, CaCO3, and calcium sulfate dihydrate, CaSO4·2H2O, to simulate the minerals calcite and gypsum, respectively. A second aqueous solution was saturated with respect to the soil, mineralogy unknown, from the RAHCO International® test site in Spokane Washington. The third solution provided the experiment control and was distilled-deionized (DI) water. A fourth set of samples were left untreated as a standard of comparison.
After the laboratory samples were molded, they were sealed in small plastic bags. Twenty-eight days after material mixing and cylinder preparation, four cylinders were submerged in each solution in closed, 250-mL polyethylene bottles. The cylinders remained submerged, agitated by magnetic stirring, for a 28-day time period. The UCS of the samples was measured together with the four untreated samples. The solid-to-liquid ratio was about 50 g of sample to 200 mL of solution or 1 to 4. The nominal sample surface area-to-volume ratio was also 1 to 4 (50 to 200 cm3).
The DI solution was removed after 28 days, evaporated, and the remaining solids weighed to determine total mass lost due to the dissolution process. The amount of recovered solid was very small, so the other aqueous test solutions, because material had been added, could not be used for this purpose.
RESULTS
The results of the laboratory test program are presented in this section. Topics include the observations made during the initial trial experiments, the relation between viscosity and slump, workability and set time results, mixture curing temperature, barrier material UCS and density, and the effect of possible leachates on barrier material stability.
Initial Tests and Observations
Several experiments were performed with a variety of aggregate, latex, and water contents, both with and without set retardant. Water contents below a water-to-cement ratio (W/C) of 35 parts water to 100 parts cement (W/C of 0.35) were too dry to adequately mix in the laboratory. Without set retardant, these mixtures set in 3 to 5 minutes. The set time and mixing efficiency of these mixtures could be increased only by increasing the water and/or latex content to undesirable, large amounts. In general, water contents greater than W/C of about 0.5 were found to diminish final strength and density. Water contents above W/C of about 0.5 were considered undesirable for this application because they usually have higher shrinkage and porosity, both of which tend to produce higher permeability in the final product. A mixture having a latex content of 15 parts (solids) per 100 parts cement, as recommended by the manufacturer, provided good mixing and handling properties. Latex concentrations above this value lowered the final product density and strength without significantly improving handling properties. Citric acid was tested as a set retardant, an additive to increase the workability and set time. Mixtures containing citric acid between 0.2 and 1.5 parts per 100 parts cement were evaluated. About 0.4 to 0.7 parts citric acid to 100 parts cement were found to be optimum for mixtures containing 15 parts latex (solids) and 35 to 50 parts water. Additions beyond 0.8 parts citric acid continued to retard the initial set time but did not significantly extend the time that the mixture could be easily worked.
Viscosity and Slump
Viscosity is the degree to which a liquid resists flow under an applied force. Slump is the distance the fluid cement mixture falls after a standard supporting cone (12-in. height) is removed. A thin, relatively low viscosity mixture would have a slump approaching the entire height of the cone, i.e., 10 to 11 in. In contrast, a slump of less than, say, 1/2 in. would indicate a very thick and virtually unworkable mixture.
However, the initial set time specification,6 in which the barrier material must be capable of supporting a 20-psi load in 15 to 30 minutes, requires that the mixture viscosity and slump must both change rapidly with time. The slump must become zero and the viscosity must become infinitely large in no more than about 30 minutes from the time of mixture preparation. On the other hand, one of the requirements for emplacement of the barrier material is that it have a slump of 8 to 10 in.
The relationship between slump and viscosity was linear within the precision of the slump measurement for a given composition. These data apply to barrier mixtures having 200 to 300 parts sand (ASTM C-33),14 to 15 parts latex, and 0.4 to 0.7 parts citric acid per 100 parts cement, and having W/C of 0.35 to 0.5. The data show that a slump of 10 in. is equivalent to a viscosity of 80,000 centipoise, and a slump of 8 in. is equivalent to a viscosity of 450,000 centipoise. The time that the mixture can be used for the BWCS application is the length of time until the slump becomes less than 8 in. or the viscosity becomes greater than about 500,000 centipoise. This is the workability time in contrast to the initial set time, which is the time required for the mixture to support a 20-psi load.
Workability Time, Set Time, and Composition
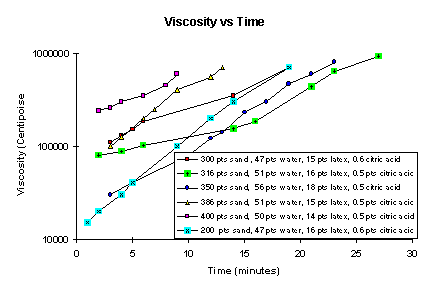
Several combinations of sand to cement from a 2:1 to 4:1 , all with about 15 parts latex, were tested. Generally, it was found that sand-to-cement ratios greater than about 3.5 required water-to-cement ratio greater than 0.5 to maintain workability. This is illustrated in Figure 1, which shows the change in viscosity as a function of time for various sand-to-cement ratios. The data indicate that a 4-to-1 sand-cement mixture has a working time of about 9 minutes compared to the 15-minute workability time of the 2-to-1 and 3-to-1 sand-to-cement ratios. Figure 2 shows the change of UCS as a function of time for the same compositions. Most mixtures have reasonable set times between 35-45 minutes. Both UCS and viscosity (or slump) constant change smoothly as a function of time. The higher 4-to-1 and 3.86-to-1 sand-to-cement mixtures also have relatively short workability times.

Figure 1. Viscosity Versus Time
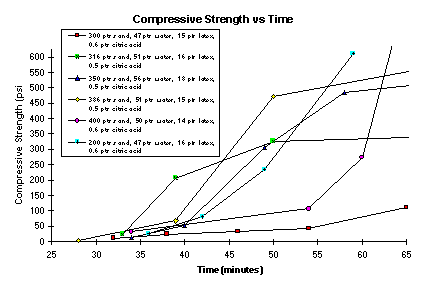
Figure 2. UCS Versus Time
The repeatability of laboratory data within the first 40 minutes is about ±5 minutes or less for a particular viscosity or UCS measurement. For example, the time to reach a viscosity of 500,000 centipoise (workability time) varies from 16 to 24 minutes and the time to reach 20 psi UCS (initial set time)varies from 36 to 45 minutes. Less water or citric acid in these mixtures would lower both the workability time and set time.
Initial set data curves were obtained for most laboratory batches exemplified in Figures 2. Longer term increase in UCS is depicted in figure 3. Initial set times varied between 4 and 12 minutes for batches that had no set retardant and 20 and 90 minutes for samples with citric acid set retardant. For samples with 0.4 to 0.7 parts citric acid to 100 parts cement and W/C in the 0.35-to-0.5 range, the set times were 20 to 45 minutes.
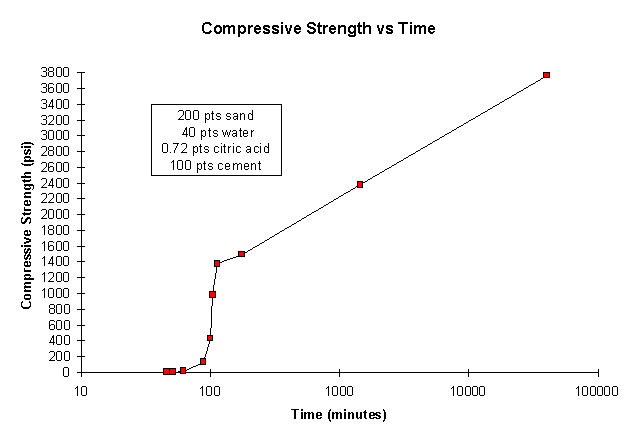
Figure 3. UCS Versus Time
Unconfined Compressive Strength and Density
As part of the sample characterization process, UCS (determined at several time periods after sample preparation) and density values were measured. UCS and density values for representative laboratory samples are shown in Tables I and II. These values are the mean of four measurements made 28 days after sample preparation unless otherwise indicated. The error is one sigma.
Table I. Composition and Physical Characteristics of the 2-to-1
Sand-to-Cement Mixtures
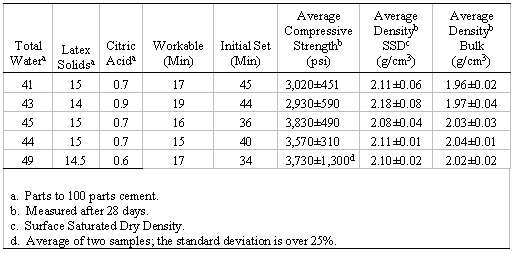
Table II. Composition and Physical Characteristics of Several Sand-to-Cement Ratios
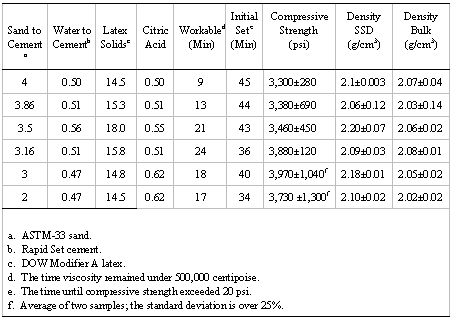
UCS versus time data are shown in Figure 3 for a composition having a 2-to-1 sand-to-cement ratio, W/C of 0.4, citric acid at 0.72 parts per 100 parts cement, and 15 parts latex (solid) per 100 parts cement. The UCS of this example was about 1,500 psi after 1.5 hours, 2,400 psi at 18 hours, and 3,700 psi at 28 days. The data indicate that the UCS would be expected to continue to increase somewhat for longer time periods. The average UCS of all samples having appropriate set time and workability properties was 3,500±500 psi measured at 28 days.
Two types of density, bulk and saturated surface dry (SSD), were measured as part of the laboratory work. Bulk density is that of the ‘as made’ concrete, air or oven dried, and was determined on all samples. SSD is the density of the sample with a dry exterior but with internal pores that are water-saturated. Bulk density values ranged from 1.9 to 2.2 g/cm3, generally about 2 g/cm3, and usually decreased slightly with an increased water-to-cement ratio.
Temperature
All cement-based materials evolve heat as they hydrate (set). This usually results in a temperature increase in the cementitious material during the set and cure processes. A requirement is that the material not reach or exceed 100° C. This specification was a safety requirement and was included in order to avoid the possibility of steam explosions during the testing of a new application of a very fast reacting exothermic material. As measured in a 3 x 6-in. test cylinder, the maximum temperature increase observed was 55° C at about 100 minutes after mixing (Figure 4). The batch composition was 200 parts sand, 44 parts water, 15 parts latex (solids), and 0.7 parts citric acid to 100 parts cement (by weight). Temperature was measured at the center of the cylinder. In comparison, 6 x 12-in. cylinders of an ordinary, general purpose Portland cement ASTM type I mixture increased in temperature from room temperature to 44° C in about 2 hours.7 As expected, the barrier materials show a sharp temperature rise during the curing process. They do not appear to pose a safety problem as a possible steam explosion source.
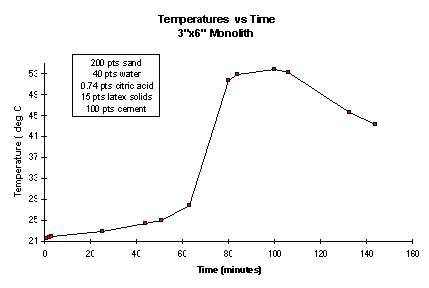
Figure 4. Temperature Versus Time
Aqueous Treatment and Material Stability
The barrier material will be applied to a waste site in an arid environment and should have chemical properties that are compatible with the soil and groundwater typical of arid climates. In such an environment, the barrier material is expected to be in the vadose zone and not in the water-saturated region below the water table. However, it is virtually certain that the barrier material will be in at least intermittent contact with aqueous solutions saturated with the mineral calcite and probably gypsum also. Gypsum can be especially troublesome for certain cementitious materials because the sulfate can react with the cement, causing cracking. Such behavior is very undesirable in a barrier material intended to have low permeability and to block the movement of contaminant materials. On the other hand, groundwaters in arid regions are typically alkaline, pH about 9, which greatly decreases the rate of cement degradation compared to, for example, more acid groundwaters of the eastern United States. The following experiments assess the affect of carbonate, sulfate, and RAHCO International test site8 soil leachates on the latex-modified Rapid Set cement.
Laboratory-prepared 1 x 2-in. molded samples and similar 1.25 x 2.5-in. cylinders core-drilled from the BWCS field material were treated to three types of aqueous solutions for 28 days: distilled-deionized water, water saturated with calcite and gypsum, and water saturated with the minerals in the soil from the RAHCO International test site in Spokane, Washington. The data indicate that there is no significant difference in UCS among the treated samples. The UCS of the control sample treated with distilled water is identical to the UCS of the carbonate-sulfate treated sample as well as the soil leachate treated sample. The data do not indicate that any reactions involving carbonate sulfate or soil leachate treated sample occurred. However all the treated samples show significantly less UCS than samples that were not treated in the solutions about 2300 versus 3600 psi for the laboratory molded samples and 2450 psi versus 4225 psi for the field core samples. This suggests that all treated samples are interacting with the water in the solutions. High standard deviations of the UCS measurements suggests nonuniform loss of sample integrity. The pH of the solutions monitored over the 28-day period. were between 10.8 and 11.8. These values typical of solutions in contact with cementitious material were usually reached in about 2 to 6 days. Mass ( both inorganic material and organic material from the latex) loss to the leachate was 1.1 wt%.
Examination, using a binocular microscope, of the samples broken during the UCS tests showed that unidentified accicular structures, having an aspect ratio of at least 50 to 1, lined the inner surface of bubble-like open spaces, probably air bubbles, within the matrix of all of the treated samples. The long axis of the features, presumed to be crystals, was perpendicular to the interior surface of the bubble. Similar open spaces were present in the untreated control samples, but no accicular crystals or other unusual features were observed. No evidence of cracking, spalling, swelling, or other features suggesting change due to the solution was observed.
The decrease in UCS is normal for latex-modified mortar5 and may be beneficial for the BWCS application provided that other properties are not degraded. Typically, water immersion causes swelling of the latex and a decrease in permeability of the barrier material as well as a loss of some UCS.5 The interaction stops at some point and is reversible if the water is removed in some manner. The decrease in UCS may indicate an increase in the ability of the material to deform without fracture, i.e., the material becomes less brittle and less likely to crack.
SUMMARY
The data indicate that the optimum BWCS barrier material compositions would be: 100 parts Rapid Set cement, 35 to 50 parts water, 15 parts latex solids (Modifier A), 200 to 300 parts concrete sand ASTM C-33, and 0.4 to 0.7 parts citric acid.
The temperature maximum of 54° C during curing suggests that the rate of heat release by Rapid Set cement is not sufficient to cause a safety issue in the field, namely a steam explosion.
The treatment of cured laboratory samples and field core samples in several aqueous solutions indicated that water decreased the UCS of the treated samples in a manner typical of latex-modified cement by 30% to 50%, accompanied by a 1% weight loss in sample mass and a slight decrease in density. Calcite and gypsum saturated solutions, as well as leachate from the test site soils, had no apparent effect on the barrier material other than the effect of water.
REFERENCES
FOOTNOTES
Prepared for the U.S. Department of Energy Under Assistant Secretary for Environmental Management DOE Idaho Operations Office Contract DE-AC07-94ID013223
a.
References herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendations, or favoring by the United States Government or any agency thereof.