LIQUID WASTE TREATMENT SYSTEM WITH INSOLUBLE TANNIN
Yoshinobu Nakamura, Wataru Shirato, Hitoshi Yamakawa, Yasunobu Tominaga,
Yasuo Nakamura
Mitsubishi Nuclear Fuel Co., Ltd.
622-1 Funaishikawa Tokai-mura,Naka-gun,Ibaraki-ken 319-11 Japan
Tel:81-292-87-8075 Fax:81-292-82-7421 E-mail:KYD02150@niftyserve.or.jp
ABSTRACT
Mitsubishi Nuclear Fuel Co., Ltd. treated radioactive liquid waste, which was generated from reconversion process and contained uranium, by co-precipitaton method with water glass. In this liquid waste treatment process, mixing of liquid waste and water glass, precipitation, aging and filtration of precipitate were implemented. Furthermore, the precipitate was treated with nitric acid and uranium was leached and recovered. Residue(water glass cake) was dried and packed into drums as low level radioactive waste. The treatment of liquid waste of 7,000m3 per year made 35 drums of solid waste. As described, the water glass system is complicated and some amount of secondary radioactive waste is stored. We have developed new liquid waste treatment system replaceable to water glass system. In the new process, liquid waste flows through columns filled with adsorbent TANNIX which is produced from tannin in an insoluble form. Uranium is adsorbed with TANNIX and TANNIX is dried and incinerated at relatively low temperature. By this process, there remains only stable uranium oxide containing small amounts of metallic impurities. The generation of metallic impurities is estimated to about several kilograms per year. This system has already replaced the water glass system in our plant and has contributed to dramatic reduction of radioactive waste.
INTRODUCTION
Since 1971, Mitsubishi Nuclear Fuel Co., Ltd.(MNF) has been fabricating PWR fuel. In UF6 reconversion lines of MNF, ADU(Ammonium Di-Uranate) process has been applied. In this process, radioactive liquid waste is generated containing uranium. Formerly, liquid waste was treated through a liquid waste treatment system with water glass. However, this system was complicated and generated secondary radioactive solid waste. Moreover, uranium recovered from liquid waste contained large amounts of impurities. To eliminate these disadvantages, we have developed new adsorbent called 'TANNIX'(trade mark). TANNIX is produced from tannin , a known precipitant for heavy metals and applied it to a new treatment system which does not generate secondary radioactive waste. In this paper, advantages of TANNIX will be introduced by comparison with the liquid waste treatment system with water glass.
LIQUID WASTE TREATMENT SYSTEM WITH WATER GLASS
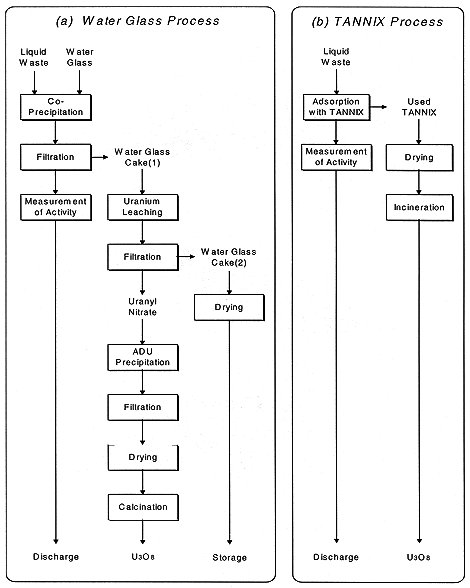
The block flow diagram of an old liquid waste treatment system with water glass is shown in Fig. 1-(a). In this system, liquid waste from reconversion lines and water glass are fed to a precipitation vessel and mixed. Uranium in the liquid waste co-precipitates with the water glass is filtered. The filtrate is measured for radioactivity and then discharged outside of the controlled area. In order to recover uranium, the water glass cake(1) is sent to a leaching vessel. Nitric acid solution is supplied and then uranium is leached from the cake(1). After filtration, the water glass cake(2) is dried and packed into drums for storage as low level radioactive solid waste. Uranium is recovered from the filtrate as ADU by adding ammonium solution. Recovered ADU is calcined and the uranium stored in the form of U3O8.

Fig. 1. Comparison of liquid waste treatment systems.
LIQUID WASTE TREATMENT SYSTEM WITH INSOLUBLE TANNIN
Insoluble tannin(TANNIX)
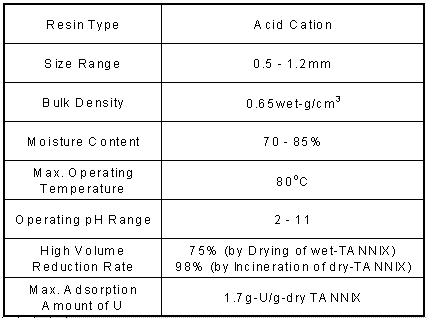
Tannin is well known such as astringent of persimmon and tea and it is complex compound of polyoxy phenol. It reacts with heavy metals in liquid but it has never been utilized for separation of heavy metals in liquid because those reaction products are soluble in water. Therefore we have transformed tannin into an insoluble form by the way of polymerizing 'wattle tannin' (condensed tannin) with aldehyde. That is, we make wattle tannin dissolve in an alkaline solution and then add formaldehyde and heat. This process produces massive amounts of insoluble tannin. It is crushed and sieved into easy to use pieces. TANNIX has excellent treatment characteristics, since the gases generated during incineration are harmless to the environment. Discharged elements only consist of carbon, hydrogen and oxygen. Volume is dramatically reduced by incineration and it has an excellent ability as an adsorbent of heavy metals in liquid. The characteristics of TANNIX are shown in Table I.
Table I. Characteristics of TANNIX
Liquid Waste Treatment System
A block flow diagram of the new liquid waste treatment system with TANNIX is shown in Fig. 1-(b). Liquid waste from reconversion lines flows through columns filled with TANNIX, and uranium in the liquid waste is adsorbed with TANNIX. As shown in Fig. 2, this equipment consists of three columns where two of them are used to treat liquid waste. In the same conventional process, after measurement for radioactivity, treated liquid is discharged to the environment. In the 3rd column, used TANNIX which has adsorbed the uranium is dried and dried TANNIX discharged at the bottom of column. Then this column is filled with new TANNIX for treating liquid waste in the next cycle. In this way, it is possible to treat liquid waste continuously by switching column operation. Discharged dried TANNIX is incinerated at low temperature and uranium is recovered in the form of U3O8.
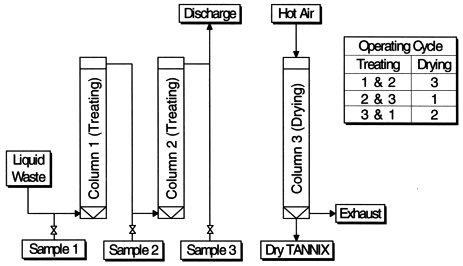
Fig. 2. Schematic flow of liquid waste treatment with TANNIX.
Results of Liquid Waste Treatment with the New System
a. Liquid waste treatment
Liquid waste is treated by the process shown in Fig. 2. Two columns are connected in series and each is filled with 0.1 m3 of 200 cm high TANNIX. Linear velocity and space velocity are fixed to 40 cm/min. and 12 /hour respectively. Liquid samples were taken at three locations ,at the inlet of the 1st column and outlet of 1st and 2nd columns about every 4 hours. The concentration of uranium in each sample was measured with an alpha scintillation counter after evaporating all liquid. The results are shown in Fig. 3 as a relationship between Volume ratio of liquid waste to TANNIX and uranium concentration. Uranium concentration of feed is between 2 and 4 Bq/cm3. Uranium concentration in the liquid which flowed through the 1st column was sufficiently under 1x10-2 Bq/cm3, which is the upper limit of the environmental release criteria, until 600 volume ratio. From 700 volume ratio, uranium concentration in the liquid at the outlet of 1st column increased and reached 1/10 of that of feed, that is defined as 'break through point' of TANNIX bed, at 1100 volume ratio. However, uranium concentration in the liquid at the outlet of 2nd column was still kept under 1x10-2 Bq/cm3. At this point, columns were switched. After switching columns, uranium concentration in the liquid which flowed through the 1st column, which was the 2nd column in preceding step, fell under 1x10-2 Bq/cm3 again. Similarly as described above, uranium concentration in the liquid through the 1st column increased from a 1800 volume ratio and reached break through point at a 2200 volume ratio. And that of 2nd column was still kept under 1x10-2 Bq/cm3. In this way, the uranium concentration is kept under the upper limit of the release criteria and the treated liquid is discharged to the environment by switching column operation.
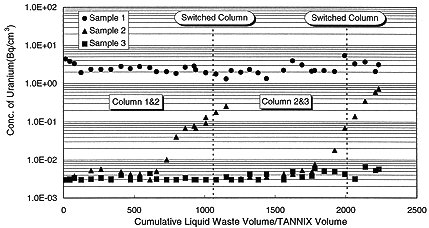
Fig. 3. Change of adsorption ability of column.
(b) Drying and incineration of used TANNIX
In order to easily discharge the used TANNIX from each column, wet TANNIX in the column was dried with hot air. Hot air was fed downward from the top of column. The volume shrank by 1/5 and flowability was improved.
Before actual incineration of the dried TANNIX, the change of weight during thermal decomposition was measured with thermobalance and impurities in the residue were analyzed. The results are shown in Fig. 4. Weight decreased as temperature increased and was remarkable over 400oC and saturated over 500oC. This shows that thermal decomposition of TANNIX finishes at 500oC. The analysis of residue shows that most of residue consists of uranium oxide and very little impurities are present. Impurities are metals which came from the rust which was mixed in the reconversion process.
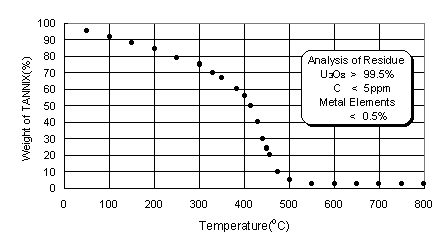
Fig. 4. Pyrolysis curve of dried TANNIX in air.
COMPARISON OF LIQUID WASTE TREATMENT SYSTEMS
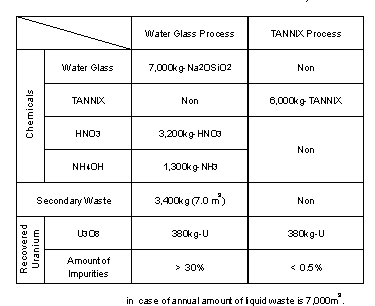
The amount of chemicals, secondary waste and recovered uranium when 7000 m3 of liquid waste is treated over a year are shown in Table II. The chemicals used in liquid waste treatment are 7,000 kg of water glass and 6,000 kg of TANNIX, respectively. In the uranium recovery process, while large amounts of nitric acid and ammonium solution are needed in water glass system, there is no chemicals in TANNIX system because uranium is recovered only through incineration. While 3,400 kg (7 m3) of used water glass are generated as secondary waste and packed into 35 drums, there is no secondary waste in the TANNIX system because of decomposition into H2O and carbon oxide gases by incineration. While uranium recovered from used water glass contains large amount of silica, the TANNIX treatment contains less than 0.5 wt% of impurities and will be recycled by simple purification. In addition, the liquid waste treatment system is drastically simplified by the TANNIX process as shown in Fig. 2 and therefore working hours are reduced to about 1/3 as compared to the water glass process.
Table II. Annual Amount of Chemicals and Secondary Waste
CONCLUSIONS
By introducing a new liquid waste treatment system with TANNIX;
REFERENCES