EMISSIONS FROM PYROLYSIS AND OXIDATIVE PYROYLSIS
EXPERIMENTS WITH ORGANIC ION EXCHANGE RESIN
Ung-Kyung Chun, Kwansik Choi, Kyung-Hwa Yang, Jong-kil Park and
Myung-Jae Song
Korea Electric Power Research Institute
Radiation Safety Group
Nuclear Power Generation Lab.
103-16, Munji-Dong, Yusung-gu, Taejon, Korea 305-380
ABSTRACT
Pyrolysis and/or oxidative pyrolysis of organic ion exchange resins and other combustible waste may be an effective pretreatment process before vitrification. To further examine these processes, organic ion exchange resins were pyrolyzed and oxidatively pyrolyzed. Volatilization of the anionic and cationic resins was observed separately for each resin as a function of temperature for pyrolysis and oxidative pyrolysis conditions. The quantity of remains or residue was found to be less with oxidative pyrolysis compared to pyrolysis-only. Between the two types of resin, cationic and anionic, the cationic exchange resin was less volatile. In addition, tube furnace experiments were performed, generally, to examine the pyrolysis of larger quantities (larger compared to the quantity of samples allowed in the Perkin Elmer 7 TGA) of cationic, anionic, and mixed resin (50% cation and 50% anion by wt.), and to examine off-gas characteristics. The cationic resin-only and anionic resin-only gravimetric results showed good agreement with the Perkin Elmer 7 TGA results. SEM pictures of the different variants of the resin (cationic, anionic, and mixed) show a different morphology for each. Off-gas data showed the presence of H2S, SO2, CO, and NO during the pyrolysis of cationic resin. CO was observed during the pyrolysis of anionic resin. The mixed resin trials showed the presence of the gases approximately at the temperatures where the gases would evolve if the results of the two different resins (cationic and anionic) were superimposed. However, the amount of hydrogen sulfide relative to the sulfur dioxide was found to increase significantly.
INTRODUCTION
Pyrolysis and/or oxidative pyrolysis of spent resin may be an important part of the pretreatment step before vitrification in a cold crucible melter (CCM). During vitrification of organic resin the carbon or other remaining residues (e.g. sulfates) from the resin may harm the performance of the cold crucible melter or the eventual stability of the final glass product (1). In addition, remaining ash or residue from the thermal treatment of spent resin not incorporated into the glass or not properly thermally treated (e.g. due to small residence time during thermal treatment) can be entrained by the off-gas flowing into the off-gas treatment system and provide significant dust levels causing unnecessary heavy loads on the off-gas treatment system(OGTS). Hence, it may be important to first reduce the amount of such waste from entering into the cold crucible melter. Pretreatment with pyrolysis and/or oxidative pyrolysis will generally provide volume reduction resulting in less amount of solid waste that needs to be handled by the CCM; in addition, the pyrolytic processes may breakdown much of the complex organics causing release through volatilization resulting in less carbon and less of other substances harmful to the CCM. Pyrolysis may be utilized with oxidation processes to further reduce the quantity of residue. In order to better understand the processes as related to the treating of resin, basic pyrolysis and oxidative pyrolysis of the resin are being examined.
Temperatures from 300 to 700°C are being utilized since these low temperatures will generally be easier to handle from technical and safety viewpoints. In addition, preliminary investigation has shown that the ash of mixed waste (e.g. mixture of various combustible waste, including resin, from nuclear reactors) pyrolyzed in this temperature range is more easily dispersed for better homogeneous mixing in the CCM glass melter and hence, provides better glass quality (2).
The investigation, the focus of this paper, is undertaken to examine some of the effects of pyrolysis and oxidative pyrolysis on unused resin as one of the beginning steps in the further investigation for the pretreatment of spent resin.
Past Work on the Pyrolysis of Organic Resin
There has been experience on resin pyrolysis. Neely (1981) of Rohm and Haas found, when pyrolyzing highly acidic cationic resins with a sulfonic group, that microporosity is created during heat treatment and that, as the temperature rises, the volume of micropores increases and the effective size of the micropores decreases (3). The micropore volume increases linearly as the skeletal volume decreases with half the volume lost by the skeleton creating new micropores and the other half appearing as shrinkage of the bead. When examining the sulfur volatiles when the resin was heated from room temperature to 300°C, he concluded that the primary form of volatile sulfur was SO2 with the general absence of SO3 and H2S.
A Hitachi group, Matsuda et. al., has done, more fundamental pyrolysis work using a quartz cylindrical tube furnace (4). They used a nitrogen flow rate of 20 ml/min. in the treatment of cation exchange resin. Each experiment was performed at a constant temperature for 2 hr. They concluded that pyrolysis of spent ion exchange resin is one of the most effective methods for reducing radioactive waste volume and for making the final waste form (e.g. in cement form) more stable. Fundamental experiments were performed to clarify the pyrolysis characteristics of anion and cation exchange resins. Residual elemental analyses and off-gas analyses showed that the decomposition ratio of cation resins with a sulfonic functional group was only 50 wt % at 600°C, while that of anion resins was 90 wt % at 400°C. Infrared spectroscopy for cation resins attributed its low decomposition ratio to formation of a highly heat-resistant polymer (sulfur bridged) during pyrolysis. Measurements of residual hygroscopicity and cement package strength indicated that the optimum pyrolysis temperatures for preventing resin swelling and package expansion were between 300 and 500°C.
Kinoshita et al. (1991) have done some fundamental studies with the treatment of ion-exchange resins such as strongly acidic cation exchange resin with the sulfonic acid functional group and strongly basic anion exchange resin with the functional group, (-CH2-N(CH3)3OH) (5). They examined the thermal decomposition behavior of ion-exchange resins in air flow with the use of a differential thermobalance and in addition, with the use of a fluidized bed incinerator equipped with copper oxide catalyst. For the differential thermobalance analyses, the resins were decomposed in three steps: first by water evaporation; second by functional group decompositon, and finally by base polymers combustion. They used a heating rate of 10°C/min. In their incineration experiments, they found copper oxide to be an effective catalyst for the treatment of ion-exchange resin.
Hence, much work in the past has been done with resins. In Korea, after the performance of a technical and feasibility study for vitrification (2), vitrification is being pursued and pretreatment possibilities are being examined. Vitrification should be an improvement over cementation since glass will, generally, provide for a more stable waste form. The glass quality is an issue, but perhaps, not as major as it could have been if there were no past experience. The higher the glass quality, generally, the higher the price. Current glass formulation exists for high level waste but for the handling of low level waste, lower qualities may be sufficient. Pretreatment may help further ease glass formulation restrictions providing a larger glass selection base, and hence, provide more economical possibilities.
Likely pretreatment methods include the pyrolysis and/or oxidative pyrolysis of the resins, the main topics of this paper. Although other likely pretreatment options may exist (e.g. combustion processes), they are not discussed or compared here. Experiments were conducted implementing a thermal gravimetric analyzer and a cylindrical tube furnace to examine ways to pretreat resin via pyrolytic processes.
EXPERIMENTAL SET-UP
PERKIN-ELMER 7 TGA.
A TGA, PERKIN-ELMER 7, that is capable of pyrolysis or oxidative pyrolysis of specimens up to 1500°C was utilized. The ultra-micro balance with a capacity of 130 mg has an accuracy of about 0.1% and a sensitivity of about 10-4 mg. A chromel-alumel thermocouple is used to examine the temperatures and is attached to the platinum crucible which contains the sample.
Tube furnace.
In addition to various Perkin Elmer 7 TGA runs, a tube furnace set-up was utilized for the pyrolysis of larger quantities of resin. The tube furnace experimentation allows us to examine some of the basic characteristics of waste at a small scale to help better design and understand what would happen in large scale experiments. In addition, even though the tube experiment processes are relatively small-scale in comparison to the real scale, they allow observations of processes on a larger scale than the Perkin Elmer 7 TGA. In addition, the bench-scale experiments also allow modifications in the experimental set-up that may not be allowed with the Perkin Elmer 7 TGA. Nitrogen is fed in from one end of the tube furnace and exhausted out the other end. The samples to be pyrolyzed or oxidatively pyrolyzed were situated midway in the tube.
The tube furnace is a quartz tube furnace that is electrically heated along its walls. It is surrounded by alumina insulation. The tube diameter is about 7 cm while the length of the tube is roughly 90 cm. Temperature control is allowed at three axial positions of the tube furnace: one midway along the length of the tube and the other two equidistant (12 cm) from the midway controller. Two K-type thermocouple probes are located midway along the length of the furnace. One probe is centered on the axis while the other probe is placed into the resin material.
Wastes.
Amberlite-77 (divinylbenzene-SO3H) and Amberlite-78 (divinylbenzene-N(CH3)3OH) are the cation and anion resins, respectively.
OPERATIONAL CONDITIONS.
PERKIN ELMER 7 TGA.
When using the Perkin Elmer 7 TGA, the sample is first dried at 100°C. Then, in general, the remaining sample of about 10 mg is heated at 5°C/min in an air and nitrogen (e.g. 25 ml/min. air and 50 ml/min. nitrogen) environment or a pure nitrogen (75 ml/min.) environment. Gravimetric analysis occurs during thermal treatment. In general, experiments were redone to examine repeatability and reliability of data.
Tube Furnace.
Batches of resin were dried in a muffler furnace for 24 hr. at 100°C. About 51% of the water evaporated at this temperature. The resin was weighed using a mass balance.
The sample of resin, around 5 g, was placed at the center of the quartz tube in a boat shaped ceramic crucible. A thermocouple probe was inserted in the resin sample and a probe was used to measure the centerline (at the midway point along the length of the tube) gas temperature. The presence of a very small thermal boundary layer was observed during the course of the experiments. Anions, cations, and a mixture of anion and cations (50% and 50% by wt.) were tested. Nitrogen was continually fed into the furnace usually at 2 l/min. The temperatures were changed by varying the oven control conditions. Temperatures were recorded for the oven wall temperatures, gas along the centerline at the midway point of the tube, and resin. The temperatures were brought up to the desired temperature and that desired temperature was maintained for roughly 1 hr. The temperature was then brought down. The resin residue was then removed at this time.
IMR recordings were also made. The IMR 3000P was used to examine the oxygen content level in the tube furnace. After about 3 minutes, the level of oxygen reached about 1 to 2%. Eventually after a few more minutes, the level of oxygen could be brought below the detection limits of the IMR. The IMR was used to scan the levels of O2, CO, CO2, SO2, NO, NO2, H2S etc. The incoming air was slightly above room temperature.
In addition, after each experiment, the quartz tube was cleaned with a solution of acetone. The acetone was generally effective in cleaning the tube. Heptane was also available for cleaning.
EXPERIMENTAL ANALYSIS
The following equipment were included for the experimental analysis: SEM for analyzing the morphology of samples; Perkin Elmer 7 TGA for thermogravimetric analysis; electronic mass balance for accurate weighings; K-type thermocouples for measurement of gas temperature and resin temperature; and an IMR 3000P for off-gas analysis.
RESULTS OF THE EXPERIMENTS.
Tube Furnace Pyrolysis Gravimetric Results.
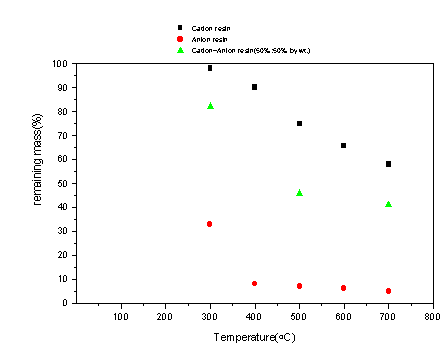
The tube furnace pyrolysis results (e.g. pure nitrogen environment) provide volume reduction data for larger resin samples (e.g. compared to the Perkin Elmer 7 TGA). Fig. 1 shows the results from the experiments for mass reduction as a function of temperature for anionic resin. The results are very similar to the results obtained from the pyrolysis runs with a Perkin Elmer 7 TGA. The cationic resin results with the tube furnace, Fig. 1, also showed similar results to those of the Perkin Elmer 7 TGA. In contrast to the anionic resin, cationic resin, when pyrolyzed, leave significant mass remains even after pyrolysis up to 700°C. Mixed resin results with the tube furnace, displayed in Fig. 1, show volatilization behavior of both the individual resins (e.g. cationic and anionic). For example, the mixed resin volatilizes at temperatures where anionic resin volatilizes and in addition, the mixed resin volatilizes at temperatures where cationic resin volatilizes.

Figure 1. Mass Loss Results for Cationic, Anionic, and Mixed Resins as a Function of Temperature When Pyrolyzed in a Tube Furnace (at 21/min. of Nitrogen).
Differences between tube furnace results and Perkin Elmer 7 TGA results, although small, may be partially attributed to differences in scale effects and possibly heat transfer effects. For example, the larger sample, will have effects of surface area exposure to gas stream, less uniform temperature distribution, etc. compared to the smaller sample.
Perkin Elmer 7 TGA Oxidation/Oxidative Pyrolysis Results with
Tube Furnace Sample.
Figure 2 shows a sample of cationic exchange resin ashes being oxidatively pyrolyzed from 100 to 900°C in the Perkin Elmer 7 TGA after the cationic had been pyrolyzed up to about 400°C in the tube furnace. It is surmised that oxidation is powerful in the reduction of residue. The net effect is that the resulting residue is generally less than in a pyrolysis-only process up to 900°C. The advantages and disadvantages for this method relative to oxidative pyrolysis-only or pyrolysis-only processes may depend not just on the mass reduction capability but also on the nature of the off-gases evolved.
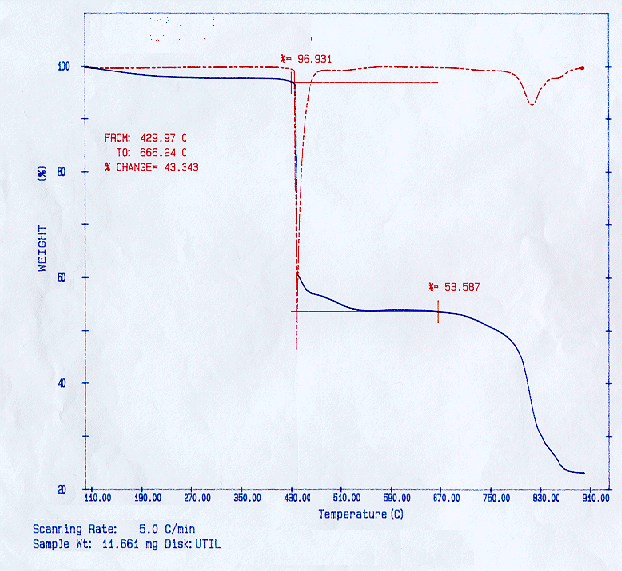
Figure 2. Perkin Elmer 7 TGA Results for Oxidative Pyrolysis (25 cc/min of Air and 50 cc/min of Nitrogen) of Ash Obtained from the Pyrolysis of Cationic Resin in the Tube Furnace (at 400 C and 21/min of Nitrogen).
Equilibrium thermochemical calculations of polystyrene by Gupta et al. showed that the pyrolysis of polystyrene at different temperatures prior to its oxidative pyrolysis provided significantly different results (6). Product gases, gaseous volume, and flame temperature is significantly affected by the pyrolysis temperature, nature and amount of the oxidant and the amount of moisture in the waste. This, too, appears to be the case with organic ion exchange resins.
Off-gas From Tube Furnace Experiments
Various off-gases were released during volatilization of the resin during pyrolysis in the tube furnace. Many of the heavier hydrocarbons/organics could be seen condensing on the walls of the glass tube. In addition, much of these organics when collected were flammable.
Off-gases were recorded with the IMR. The IMR was able to record the following effluents: H2S, SO2, CO, NO, NO2 and O2. Of these effluents, for anionic resin studies, only the off-gas release of carbon monoxide was detected. For the cationic resin studies, only sulfur dioxide, hydrogen sulfide, NO, and carbon monoxide were detected. By the examination of the off-gas characteristics, some of the fundamental behavior of the resin during pyrolysis may be observed and some conclusions surmised.
Cationic Resin.
For pyrolysis of cationic ion exchange resin, the off-gas results as detected by the IMR are displayed in Fig. 3. The detection of SO2 by the IMR began around 350°C followed by dramatic increases in SO2 level concentrations resulting in two peaks around 410 and 440°C. The SO2 level dramatically drops after the 2nd peak to very low levels by about 450°C. The CO starts to become detected at about 520°C. There are eventually two peaks in CO where one is centered around 630°C and the other around 720°C. The real second peak for CO still requires further investigation since the maximum temperature reached during the experiment was only about 720°C and a further increase in the temperature would likely have resulted in higher levels of CO.
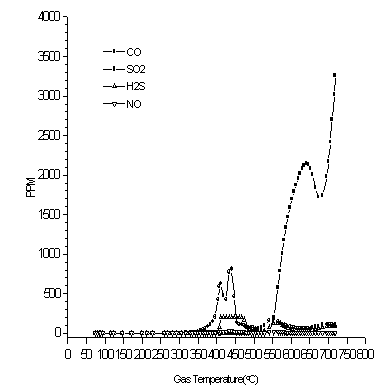
Figure 3. As Detected by the IMR, Off-gas Releases as a Function of Temperature for 4g of Cationic Exchange Resin Pyrolyzed in a Tube Furnace (at 21/min of Nitrogen).
Matsuda et al. note that they found two different forms of sulfur bridges in the residue in their experiments (4). One was a sulfur bridge and the other was a SO2 bridge. Using X-ray photoelectron spectroscopy (XPS), they found that there were two sulfur related peaks. One had a value of 167.9 eV peak measured for diphenyl sulfone (C6H5)2SO2 and another at 164.0eV for phenyl sulfide polymer (C6H5S)n. They surmised that the tendency was first, for the formation of SO2 bridges followed by SO ones. It is possible that the two peaks in the SO2 profile in the IMR off-gas analysis are related to the different sulfur bridge-type formation tendencies. The first peak in the SO2 may occur during the building of SO2 bridges while the second peak may result during the formation of S bridges. As temperatures rise and the base polymer composed of these two different bridge types somewhat breaks down, the difference in the breaking of the different bridges may result in the formation of two CO peaks. It is possible that the CO peak at the higher temperature may correspond to the breakdown of the S bridges. Matsuda et al. noted in their XPS analysis that at 500°C, there was a disappearance of the diphenyl sulfone and that there was some release of hydrocarbons (4). It is very possible that the first CO peak indicates the release of the hydrocarbons and perhaps, the breakdown of much of the diphenyl sulfone. The off-gas results do not show significant SO2 release at this time – hence, the sulfur remains in the residue, perhaps forming more phenyl sulfide. Later, at higher temperatures, we start to see the CO levels rise again and this may be associated with the breakdown of the base polymer and likely, some of the phenyl sulfide polymer. During this time, the CO levels are higher than during the assumed breakdown of the diphenyl sulfone and hence, the hydrocarbon breakdown is likely higher at this time. This agreed with the Perkin Elmer 7 TGA results.
H2S and NO.
Other gases H2S and NO that were detected by the IMR were small compared to the SO2 or CO. But if the quantity of resin treated is large enough, these gases may be significant. The hydrogen sulfide generally appeared during the release of sulfur dioxide.
Anionic Resin.
It is interesting to note that the IMR detected CO that had two peaks. By examining the IMR results along with Perkin Elmer 7 TGA data, the start of the CO level generally corresponded to where there was a change in the mass of the anion sample. The two peaks indicate that there are two regions where the mass is changing due to the release of volatiles. By studying the IMR data along with the Hitachi group’s work with anionic exchange resin, it is surmised that the first peak of the CO occurs during the breakdown of the functional group and the release of amine gas while the second CO larger peak occurs during base polymer breakdown.
Mixed Resin.
The IMR detected off-gas profiles as shown in Fig. 4 show for the most part, results that could have been obtained from a superposition of the gases released from the separate cationic and anionic resins tests. There are some differences as would be suggested from the new morphology arising from the mixture of resins. The level of hydrogen sulfide has appeared to increase relative to the sulfur dioxide.
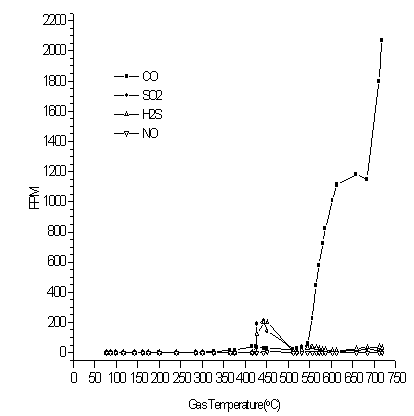
Figure 4. As Detected by the IMR, Off-gas Releases as a Function of Temperature for 4g of Mixed Resin (50% Cationic and 50% Anionic Resin by wt.) Pyrolyzed in a Tube Furnace (at 21/min of Nitrogen)
DISCUSSION
The pyrolysis of resin in the tube furnace experiments showed the presence of sulfur gases. Pyrolysis followed by some oxidation process may change the form of the sulfur gases but is not likely to remove it. Because of the significant amount of sulfur gases, there is the likely need for acid gas scrubbers in the off-gas treatment system for the pretreatment system. Any significant amount of sulfur remaining in the ash will also pose problems for the CCM. The sulfur can create a corrosive environment and may, along with carbon, harm the final quality of the glass form.
CONCLUSION
The study of complex processes, generally requires, the understanding of more basic or fundamental processes. The attempt to examine the pretreatment of spent resin was started with the examination of unused resin with a Perkin Elmer 7 TGA and then, with a cylindrical quartz-tube furnace via pyrolysis and oxidative pyrolysis. Much of the data obtained from this research can be substantiated by the results obtained by past researchers.
The volatilization of each of the anionic, cationic, and mixed resins was observed as a function of temperature for certain pyrolysis and oxidative pyrolysis conditions. The quantity of remains or residue was found to be dependent on the process: generally, oxidative pyrolysis processes resulted in less remaining residue. The cationic exchange resin, as expected, was in general harder to volatilize. The anionic exchange resin during pyrolysis showed the start of significant volatilization at the temperatures of about 220°C and then again at about 350°C. The cationic resin started its significant volatilization at about 410°C during pyrolysis. Mixed resins were pyrolyzed and oxidatively pyrolyzed. The mixed resins during pyrolysis showed volatilization behavior that included the behaviors of the cation-only and anion-only trials.
Tube furnace experiments were performed to examine the pyrolysis of larger quantities of resin compared to the Perkin Elmer 7 TGA. Cationic, anionic, and mixed resins (50% anionic and 50% cationic resins by wt.) were pyrolyzed. The gravimetric results showed good agreement with the Perkin Elmer 7 TGA results. SEM pictures of the cationic, anionic, and mixed show a different morphology for each. Off-gas data showed the presence of H2S, SO2, CO, and NO during the pyrolysis of cationic resin. CO was observed during the pyrolysis of anionic resin. The mixed resin trials showed the presence of the gases approximately at the temperatures where the gases would evolve during non-mixed trials. However, the amount of hydrogen sulfide was found to increase significantly relative to the sulfur dioxide.
Further pyrolysis and oxidative pyrolysis pre-treatment investigaton is required and will be pursued. The effect of changing the percentage of each of the resins in the mixed resins, changing the amount of oxidants during pyrolysis, catalytic effects of various metals, presence of other combustibles, etc. will be investigated.
ACKNOWLEDGEMENTS.
Miss Mi Kyoung Kwon of KEPRI is duly acknowledged for her technical services. Mr. Serge Merlin of SGN (France) is acknowledged for his suggestions.
REFERENCES