LEACHABILITY OF RADIOACTIVE NUCLIDES FROM 200 L
BITUMINIZED WASTE FORM
Jae-Won Lee, Young-Gerl Ryue, Ki-Hong Kim
Korea Atomic Energy Research Institute
P.O.Box 105, Yusongku Taejon, 305-600, Korea
ABSTRACT
The leachability of the full-scale bituminized waste form (200 L) containing 15 wt% ground spent ion- exchange resins and 5 wt % sodium nitrates produced from Radioactive Waste Treatment Facility (RWTF) in Korea Atomic Energy Research Institute (KAERI) was investigated by static leach test at Radioactive Waste Form Characterization Facility (RWFCF). After one year leach test, the effective diffusion coefficient was determined using semi-infinite diffusion model. The release of Cs-137 showed the two-step processes that the first step was a slow release and the second step was a rapid release due to the swelling of bituminized ion-exchange resins by the uptake of water. These leaching behaviors were efficiently explained by diffusion model. The effective diffusion coefficient of Cs-137 was 3.61 x 10-13 cm2/sec in a slow release region and 3.40 x 10-10 cm2/sec in a rapid release region. However, the release of Co-60 up to 180 days was controlled by diffusion with effective diffusion coefficient of 1.45 x 10-14 cm2/sec, whereas after 180 days it showed a complex leaching mechanism with consistently reducing of cumulative fraction release.
INTRODUCTION
Low level liquid radioactive wastes and spent ion-exchange resins produced at research facilities and laboratories in Korea Atomic Energy Research Institute (KAERI) were immobilized by bitumen at Radioactive Waste Treatment Facility (RWTF). Bituminized waste forms of 21 drums were produced since normal operation of RWTF in 1991. Bituminized waste forms in 200 L drum were routinely prepared in the formulation of 15 wt% ground spent resins and 5 to 10 wt% salts.
In Korea, the disposal of low radioactive waste form in rock cavern had been suggested. In rock cavern disposal site, it was expected that flow of ground water was generally very slow and static. Then static leach test method was introduced to demonstrate the stability and the leachability of full-scale bituminized waste form.
WASTE FORM AND LEACH TEST
Waste Form
A full-scale bituminized waste form containing 15 wt% ground spent ion- exchange resins and 5 wt% sodium nitrates produced from RWTF in KAERI was used in this leach test. Spent resins in composition of cation-exchange resin (43.5 vol%) and anion-exchange resin (56.5 vol%) were produced from the pool water treatment and sodium nitrates from the decontamination from at Post Irradiation Examination Facility (PIEF). Spent ion-exchange resins were ground into less than 100 m m by wet grinder (Fryma stone mill) at RWTF. Mixture of ground spent ion-exchange resins and sodium nitrates were adjusted to pH = 7 with sodium hydroxide. Waste form in 200 L drum (DOT-17H) was prepared by mixing Mexphalte 60/70 (shown in TABLE I.) with those wastes through the thin film evaporator (LUWA 150f ). Total amount of Cs-137 and Co-60 contained in waste form was 40.142 and 42.617 mCi, respectively.
Table I. Properties of Mexphalte used in KAERI Bituminization Process
|
Item |
Standard Method |
Result |
||
|
Penetration(a) |
ASTM-D5 |
64(1/10 mm) |
||
|
Softening point |
ASTM-D36 |
48.0 ° C |
||
|
Flash Point |
ASTM-D92 |
326 ° C |
||
|
Ductilty |
ASTM-D113 |
>150 cm |
||
|
Thin Film Oven Test |
Penetration(b) |
ASTM-D1754 |
ASTM-D5 |
71.8 %(b/a * 100) |
|
Ductility |
ASTM-D113 |
> 150 cm |
||
|
Solubility in Trichloroethane |
ASTM-D2042 |
99.82 % |
||
Leach Test Apparatus
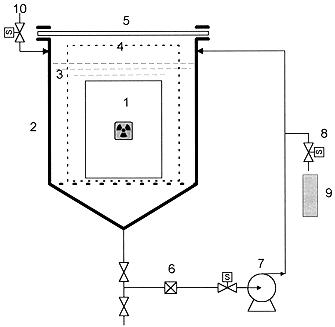
Leach test apparatus was schematically drawn in Fig.1. It consisted of leaching vessel of 600 L, strainer of 100 m m for the protection of pump from suspended particles, low flow rate circulation pump of 1 L/min for sampling of homogeneous leachant, and timer-attached solenoid valve for sampling of leachant. Materials of all components in contact with leachant were made of stainless steel 304. Transparent acrylate board at upper part of leach vessel was installed in order to monitor physical change of waste form by remote-controlled camera. For charging of waste form into leach vessel and discharging, waste form handling device was inserted in leach vessel. In order to sample a given volume of leachant, a control timer was attached to sampling solenoid valve. Strainer was replaced once a month.

Fig. 1. Schematic Diagram of Leach Test Apparatus
1. Waste Form and Drum
2. Leaching Vessel
3. Leachant
4. Waste Drum Handling Tool
5. Acrylate Board
6. Strainer
7. Circulation Pump
8. Sampling Valve
9. Sampling Bottle
10. Leachate Filling Line
Leaching Test
The static leach test was conducted in hot cell at Radioactive Waste Form Characterization Facility (RWFCF) in KAERI. Lid-open waste drum after decontamination of drum outer surface was immersed into leach vessel, which contained 257 L of deionized water equivalent to 10 times of the surface area exposed in leachant. Deionized water with an electrical conductivity of less than 5 m mho/cm was used as leachant. Test temperature was constantly kept at 20oC in hot cell. Leachate of 500 ml was periodically sampled through timer-attached solenoid valve during circulating of leachate by a low flow rate pump. After analysis of radioactivity, leachate was refilled into the leach vessel. The amount of Cs-137 and Co-60 in leachate was analyzed by g -ray spectroscopy using HPGe 25190-P detector.
LEACHABILITY OF RADIONUCLIDES
Release of Cs-137
Cumulative fraction release of Cs-137 versus square root of leaching time was shown in Fig.2. The leaching curve showed a slow release(in region I) and a later, more rapid release(in region II). The mechanism of leaching of radioactive nuclides from bituminized waste forms was interpreted by various leaching processes such as diffusion, solubility, and diffusion plus absorption [1,2]. In the region I, the leaching was characterized by its dependence on the rate that water penetrate the bitumen itself. The duration of the slow release was controlled by the thickness of bitumen layer at the surface of the waste form and by the physical characteristics of that layer, such as microcracks and voids due to shrinkage. In the case of region II, the leaching was characterized by a distinctive increase in the leach rate due to dissolution or swelling of the waste within the bitumen matrix [3,4].
The two-step release in Fig. 1. was explained due to the swelling of the bituminized ion-exchange resins by the uptake of water. Two leaching curves were linear with leaching time in Fig. 1. Effective diffusion coefficient of Cs-137 was determined by semi-infinite diffusion model as follow;
![]()
where S an is the total amount of radioactivity released in all leaching periods up to time, t, A0 is the initial amount of radioactivity; V is the initial volume of the waste form, S is the initial surface area of the waste form, De is the effective diffusion coefficient in a porous medium, t is the cumulative leaching time, and b represents the fraction of the initial radioactivity released from the surface area of waste form. The effective diffusion coefficient were calculated to be 3.61 x 10-13 cm2/sec for region I and 3.40 x 10-10 cm2/sec for region II, using curve-fitting. The cumulative fraction release was 3.58 x 10-5 at 222 days in the end of region I and 1.66 x 10-4 at 364 days. The release rate was 2.52 pCi/cm2× day in the slow release region and 32 pCi/cm2× day in the rapid release region.

Fig. 2. Cumulative Fracton Release of Cs-137
Release of Co-60
Cumulative fraction release of Co-60 versus square root of leaching time was shown in Fig.3. The release of Co-60 from bituminized waste form was controlled by two complex processes. At the first step (region I), the cumulative fraction release of Co-60 was linear with the square root of leaching time. A diffusion model could be applied to interpreting the leaching process. Effective diffusion coefficient of Co-60 was 1.45 x 10-14 cm2/sec up to 180 days, which was lower than that of Cs-137. The cumulative fraction release was 6.96 x 10-6 and the release rate was 0.64 pCi/cm2× day. However, at the second region the release of Co-60 was reduced with increasing leaching time. This behavior may be guessed due to interaction of Co-60 in the leachate with the corroded products of carbon steel drum.

Fig. 3. Cumulative Fraction Release of Co-60
pH Change
Duration of one year leaching test, results of pH analysis was shown in Fig.4 with range of 6 to 7.1. pH was continuously rising with increasing leaching time. It indicated that release of radioactive nuclides and NaNO3 continued.

Fig. 4. PH Change with Leaching Time
CONCLUSION
The release of Cs-137 from the bituminized waste form was controlled by two step processes due to the swelling of bituminized ground ion-exchange resins by the uptake of water. The leaching of Cs-137 was explained by diffusion model. After swelling, the effective diffusion coefficient increased to about a thousand times than that that of no swelling. Release of Co-60 was suggested to be very complex in this leaching system. The growth of swelling on the upper surface of waste form was observed. This leach test will be continued for several years in order to evaluate the long-term leachability.
SYMBOLS
KAERI: Korea Atomic Energy Institute
RWTF: Radioactive Waste Treatment Facility
RWFCF: Radioactive Waste Form Characterization Facility
PIEF: Post Irradiation Examination Facility
REFERENCES