ZEOLITE WASTE FORMS SYNTHESIZED FROM SODIUM BEARING WASTE AND METAKAOLINITE
Darryl D. Siemer, Michael W. Grutzecka ,
Della M. Roy and Barry E. Scheetz
Materials Research Laboratory
The Pennsylvania State University
University Park, PA 16802 USA
ABSTRACT
The object of this research was to demonstrate that US defense-type reprocessing waste could be converted into insoluble cryptocrystalline mineral-assemblage "waste forms" using relatively mild hydrothermal processing conditions. The process modeled by this study is a marriage of Hanford's "Clay Reaction Process" with Oak Ridge National Laboratory's (ORNL's) FUETAP technology. The "hydroceramic" materials produced were physically strong and much more leach resistant than typical radwaste "grouts."b They also out-performed DOE's official HLW quality control standard on the PCT test.
INTRODUCTION
By the late 1960's Hanford scientists had come to realize that the greatest technical challenge to making defense-type HLW suitable for disposal (i.e., converting it to a form that would be insoluble in a burial site's groundwater) was posed by the massive amounts of intrinsically soluble material (mostly sodium salts) in it and also that glass-making on the required scale would prove to be prohibitively expensive. Consequently, they developed chemical solidification processes1,2 similar to those commercially employed to make zeolites to convert this waste into insoluble aluminosilicate framework minerals having cage-like arrays of channels and cavities, e.g. zeolites and feldspathoids (sodalites and cancrinites). These workers stressed that these minerals tended to form naturally (i.e., would be stable) in North America's more appropriate potential repository sites3-7 and also that processes capable of rendering alkalis insoluble would make other elements (toxic, non-toxic, radioactive or otherwise.) even less soluble. In other words, solidification processes able to fix sodium would be "conservative" with respect to a waste's hazardous components.
A drawback of those processes was that their immediate products consisted of tiny clay-like particles which had to be separated from reaction liquors and consolidated into transportable monoliths by other means. The purpose of the current work was to demonstrate that, if Hanford's "Clay Reaction Process"8 were to be implemented via ORNL's almost-equally venerable FUETAP process,9 all reactants in any given batch could be converted into a low-solubility, "canned" monolith in a single step. The work described below builds on earlier work by the authors.10,11
EXPERIMENTAL
Most of the specimens were made with a calcined kaolinitic clay (containing metakaolinite) produced by Ashgrove Cement Co. See Table I for its composition. After heat treatment the X-ray diffraction pattern of the metakaolinite is dominated by an amorphous "hump" centered at approximately 22° 2q and peaks representative of minor amounts of opaline cristobalite and quartz. Because the calcination process disrupts the clay's crystal structure, the metakaolinite possesses high pozzolanic reactivity (will react with lime water and alkaline solutions in general). Numerous authors7 have shown that metakaolinite could be reacted with sodium hydroxide (NaOH) to produce zeolites. This suggested that this material should also be suitable for converting high-sodium radwaste into both zeolites and feldspathoids such as sodalite and cancrinite.
Table I. Composition of Troy Creek Metakaolinite

Various simulants were prepared, ranging from simple to quite complex. Most were prepared by dissolving aluminum metal in a concentrated NaOH solution - usually at the same Na:Al atom ratio (3:1) present in the Idaho National Engineering and Environmental Laboratory's (INEEL) as yet uncalcined sodium-bearing waste (SBW). In some instances, sodium nitrate and/or sodium carbonate salts were substituted for some of the NaOH in an effort to gauge the effect of common anionic species on waste form performance. Other simulants were made of nitrate salts (Na, K, Ca, Fe, Al, & Zr) plus nitric and hydrofluoric acids. These were meant to emulate both the liquid waste stored in INEEL's HLW storage tanks (i.e. solutions of salts and acids) and the calcines stored in its binsets (low-fired calcines made from solutions of salts, acids, etc.). Unlike the simple simulants, these mixtures were first sugar-calcined at 500ECc to destroy most of the nitrate. Generally, the proportions of simulant to pozzolan used were such that the overall alkali:aluminum:silicon atom ratio in the composite (grout) were close to those needed to form zeolites, sodalites and/or cancrinites (1Na:1Al:>1Si atom ratio). When substantial amounts of water-soluble sodium salts were present, the proportion of NaOH to metakaolinite was usually adjusted so that a product having the generic "sodalite" formula [(NaAlSiO4)3 NaX where X can be SO4=, NO3, Cl-, etc.] could be produced. We have called this strategy the "sodalite rule of thumb."
In all cases, water additions were limited to the minimum needed to make a grout that would settle into molds being vibrated with an engraving tool. After an initial set had been achieved by exposure of the samples to atmospheric-pressure steam, the molds were put into a homemade autoclave [made from 37 mm i.d. mild steel pipe and two 76 mm flanges] along with water and "cured" in an oven (usually @ 200° C) for several hours. During curing, the molds were placed on a platform above the water, not immersed in it. Cured specimens were taken from the autoclave and put back into the oven to remove unreacted pore waterd.
The products were characterized by X-ray diffraction and SEM analyses plus three of the leach test protocols commonly used by today's radwaste management community; the ANSI-16.1, the PCT, and the TCLP. The ANSI-16.1 measures the mobilities (D in units of cm2/s) of individual components of a material under leaching conditions that discourage back reactions/saturation. It was performed using the "standard" approach12 with some adjustment of the recommended time intervals to accommodate work schedules.
The PCT indicates a material's gross solubility under leaching conditions that tend to encourage saturation/back-reaction. It was also performed using a modified version of the "standard" approach13. The weight ratio of the leachant (distilled water) to sample powder was usually 10:1 (the recommended ratio) and the temperature was ~95 ° C (the boiling point of water at INEEL) instead of the recommended 90° C. This test is of special relevance because the USA DOE officially bases its HLW "Waste Acceptance Product Specification" (WAPS) on a material's seven-day PCT performance relative to that of a benchmark, EA glass.
The EPA's TCLP test14 involves the leaching sub-nine mm sample fragments for 18 hours in 20 times as much (by weight) room temperature 0.1M pH 4.93 acetate buffer solution.
RESULTS AND DISCUSSION
Superficially, the bulk of the hydroceramic specimens made during the course of this project appear much like ordinary portland cement (OPC)-based concretes in that they are buff-colored, moderately-hard solids possessing bulk densities (1.2 to 1.7 g/cc, dry-basis) well below those of natural minerals composed of the same elements. This suggests that they too possess a good deal of porosity. However, the characterization tests showed that they were actually quite different. Most of this paper is devoted to a detailed description of two examples.
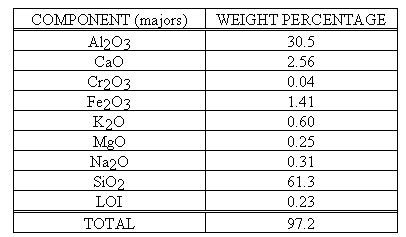
Figure 1 represents the X-ray diffraction pattern of a hydroceramic made from a simple sodium aluminate-sodium hydroxide simulant and metakaolinite (2 hr cure at 200° C) and Figure 2 represents its microstructure. The "stick figures" below the pattern represent the position of peaks for quartz, hydroxysodalite and zeolite A15 . In addition, the X-ray diffraction pattern also indicates that not all of the sample has crystallized, it still contains a sizable amorphous "hump" centered at ~20° 2q. This represents a 2° shift from metakaolinte's characteristic peak at 22° 2q. The broadness of that hump seems to indicate that most of the metakaolinite in the sample has reacted, but the highly disorganized nano-sized crystallites that formed have not yet developed a sufficient degree of long range order needed to be detected using X-ray diffraction analysis. The crystalline phases that formed include hydroxysodalite [Na8Al6Si6O24(OH)2· 4H2O] and zeolite A [Na12Al12Si12O48· 27H2O]. The quartz present in the sample is a remnant of the quartz originally found in the metakaolinite. The microstructure of the hydroceramic (Fig. 2) indicates that the platy and spherical crystallites tend to interlock much like the calcium silicate hydrate (C-S-H) found in portland cement. Because crystalline zeolites and feldspathoid minerals (sodalites, cancrinites) possess densities greater than 2.0 g/cc, the bulk density values obtained earlier are consistent with the observed microstructure.
The composition of the raw liquid simulant (Table II) and that of the sugar calcine (30 wt%) plus metakaolinite (70 wt%) mixture that were used to prepare our "complex" SBW hydroceramic example is given in Tables II and III, respectively. It is important to mention here that the sugar used to facilitate low-temperature calcination also converted most of the sodium in the sample to sodium carbonate. The grout was autoclaved at 200° C for 3.5 hours. Figures 4 and 5 represent its phase and microstructure.
Table II. Composition of Liquid Simulant*
|
Table III. Composition of Specimen*
|
The phases present in the sample are hydroxysodalite [Na8Al6Si6O24(OH)2· 4H2O] and cancrinite [(Na6Ca2)(Na6Al6Si6O24(CO3)2· 2H2O]. The pattern is generally more crystalline, and as a result its microstructure is slightly different than that of the hydroceramic described earlier. The crystals that form in this sample are generally larger and more spherical in appearance having ,what has been called, "balls of wool" morphology. Note that these "balls of wool" seem to be associated with the surfaces of thicker and less prominent platy samples.
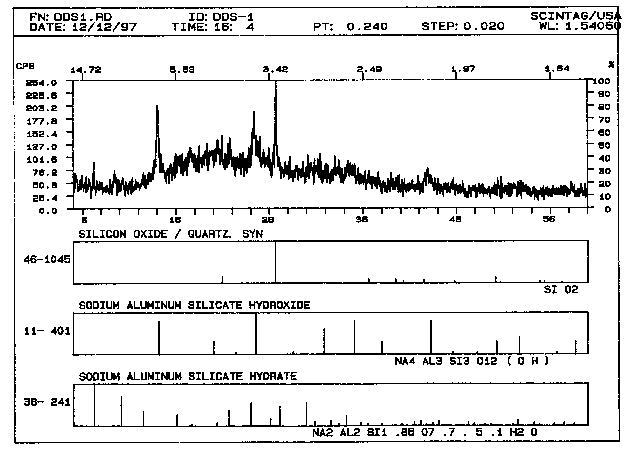
Fig. 1. X-Ray Diffraction Pattern of Hydroceramic Made with Sodium Aluminate, Sodium Hydroxide and Metakaolinite Reacted At 200° C For 2 Hours. the Stick Figures Represent the Positions of Peaks for Quartz, Hydroxysodalite and Zeolite A (JCPDS).
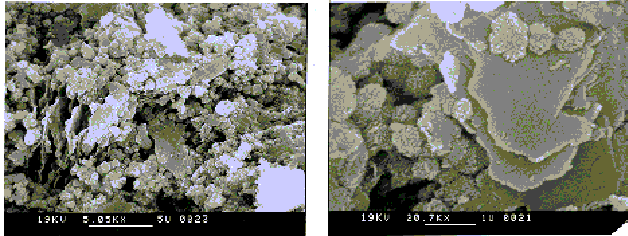
Fig. 2. SEM Images of Hydroceramic Made with Sodium Aluminate, Sodium Hydroxide and Metakaolinite. This is a Broken Surface. Original Magnifications are 5,000x And 20,000x, and Bars Are 5 and 1 mM Long, Respectively.
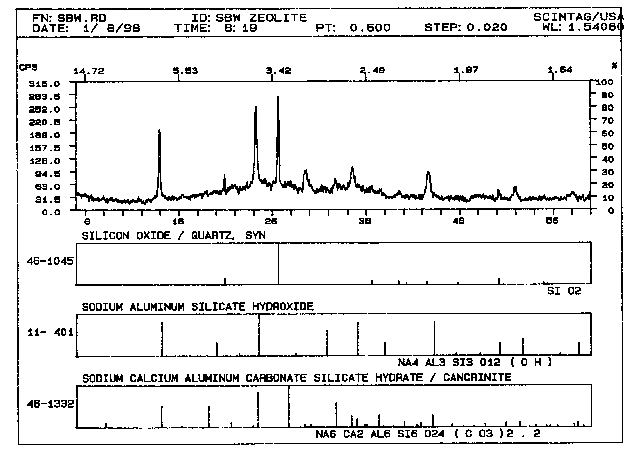
Fig. 3. X-Ray Diffraction Pattern of Hydroceramic Made with Na2CO3 Containing Sugar Calcined SBW and Metakaolinite Cured At 200° C For 3.5 Hours. The Stick Figures Represent the Positions of Peaks for Quartz, Hydroxysodalite and Cancrinite (JCPDS).

Fig. 4. SEM Images of Hydroceramic Made with SBW and Metakaolinite. This is a Broken Surface. Magnifications are 5,000X And 20,000X, and Bars are 5 and 1mM Long, Respectively.
The reason that hydroceramics tend to form salt-substituted hydroxysodalites and cancrinites rather than zeolites is due to the fact that the minimal amount of water used to make these grouts causes initial soluble salt/hydroxide concentrations in the aqueous phase to be extremely high. Breck7 suggests that, in the presence of sodium hydroxide, dilute aluminosilicate gels (containing 90-98 mole % H2O ) readily form zeolites (A, X, Y), while more concentrated systems (containing 60-85 mole % H2O) tend to form hydroxysodalite instead. The simple-simulant example of Figs. 1 &2 contained a trace of zeolite A. However, this zeolite is metastable under these conditions and will alter to hydroxysodalite with time. Breck also suggests that sulfate and halogen ions tend to accelerate conversion of zeolite A to hydroxysodalites. This would explain why our calcined SBW sample did not contain zeolite A. While it may have initially formed, it then converted to salt -substituted hydroxysodalite and/or cancrinite.
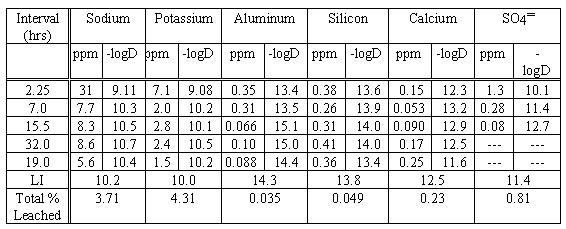
Table IV gives ANSI-16.1 results for a hydroceramic made from the sugar-calcined SBW (Tables II and III). This sample was autoclaved at 200° C for 3.5 Hours.
Table IV. ANSI Leach Data: SBW Specimen Autoclaved at 200° C for 3.5 Hours
*
the piece leached had a geometric surface area of 3.8 cm2 and weighed 0.67 gramThis specimen was fairly typical in that the leach indices (LI = -log10 of the mean of all-interval D's) of all of the metals were high ¾ those of Na & K abnormally so. It was also typical in that a usually soluble anion (sulfate) had apparently been "fixed" as well. However it differed from most of the specimens in that its bulk density (~1.18 g/cc), physical strength, and PCT performance (9.9% of the sodium leached) were well below the norm. The reason for this is that because both carbonate and aluminate are much less basic than is "free" hydroxide, they did not dissolve/mobilize the pozzolan's primary network-forming components (silica & alumina) as effectively. It was also unusual in that the predominant anion in the leachates was carbonate not hydroxide. Most specimens leached via hydrolysis; i.e., the majority of the anionic material in the leachates was hydroxide ion (probably) derived from the dissociation of water.
Another piece of this specimen was subjected to the TCLP test - see Table V. The intrinsic insolubility of most silicates plus the fact that sodium-zeolites and feldspathoids serve both to buffer solutions and scavenge metals from them explains why hydroceramics always pass this test. It should be noted that elements apt to be present as anions (Cr, Se, and As) are also fixed.
Table V. TCLP: 30% Calcined "Sodium Bearing Waste" Specimen
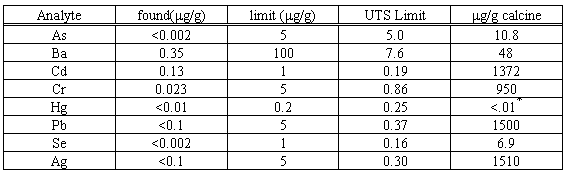
*Mercury was not added to the original liquid simulant because it would have been lost during calcination. In a real calciner, mercury would be recovered from the offgas.
Table VI compares seven-day PCT performance of several radwaste-type glasses with those of seven different hydroceramics (HCs). The glasses include DOE's benchmark, EA glass, plus two others "representative of compositions to be used at SRP's Defense Waste Processing Facility's vitrification plant." 16 The first two hydroceramics listed were made with differing amounts of the before-mentioned 3:1 Na:Al simulant and Troy Creek metakaolinite to make materials with different Na2O "waste loadings," 16.7% for the first and 10.8% for the second (dry basis). The third HC was made with a 3:1 mole-wise mixture of sodium hydroxide and sodium nitrate (the "sodalite composition" ratio), the same metakaolinite , plus some quartz sand "aggregate" (total sodium = 12.6% Na2O). The fourth and fifth HCs were made with synthetic calcines having the compositions of the bulk of INEEL's existing reprocessing wastes, "alumina" and "zirconia" calcines. The dry-basis composition of the fourth was 38% alumina calcine, 26.7% blast furnace slag cement, 26.7% diatomaceous earth (pure silica), plus 9.3% Na2O added as a water solution of NaOH (13.1% Na2O total). The fifth HC was 46% zirconia calcine, 46% clay and 8% Na2O added as sodium hydroxide. The sixth HC is the same 30% sugar-calcined SBW specimen described in Tables II-IV of this paper. The seventh and last HC was made with a metakaolin possessing a greater proportion of alumina to silica.
The main point of Table VI is that it demonstrates that hydroceramics are less water soluble than are typical radwaste-type glasses. In spite of the fact that the intrinsic porosity of hydroceramics gives sample powders made from them far greater surface areas (the BET area is over a thousand fold higher) than those made from these glasses, the latter lose a higher fraction of their alkalis (accompanied, of course, by an equivalent amount of anionic material - primarily borate and silicate) over the usual duration of the PCT. In practice, PCT leachates of hydroceramic-type materials reach steady-state within two or three days - nothing more dissolves regardless of how long leaching is extended past that time. On the other hand, in extended-duration PCT tests, EA glass continues to dissolve until virtually all of the intrinsically soluble materials (alkali metals and borate) in it goes into solution. This is not really surprising: in order to make vitrification "practical" (i.e., easy-melting, low viscosity glass), DOE's glass-makers deliberately separate/discard the component of most DOE-type radwaste that would make their glasses more durable (aluminum) and replace it with lithium and boron.
Table VI. Comparison of Sodium Leachabilities
from Radwaste Glasses and Hydroceramics in 7-Day PCT
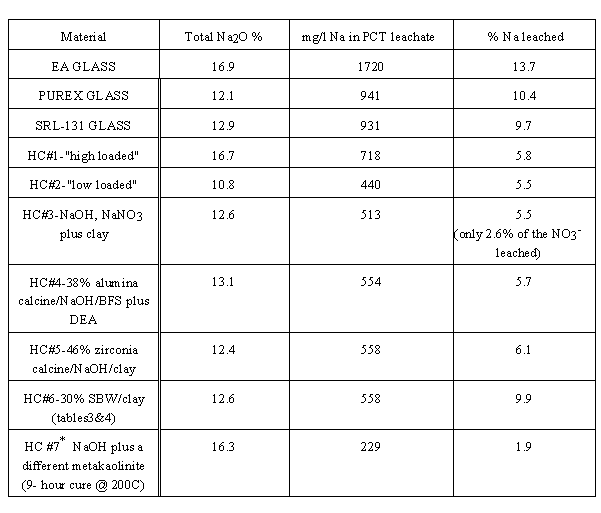
*the sodium ANSI-16.1 leach index of this specimen was 11.6.
Another point exemplified by the performance of the seventh HC listed in Table VI (the only example that had been made with a different metakaolinite) is that the two specimens detailed in this paper do not represent "optimum" hydroceramics. The real potential of this technology can be realized only if/when today's decision-makers become willing to fund scientific research on alternative HLW waste forms.
CONCLUSIONS
The primary conclusion to be drawn from both this and the prior research is that there appears to be no valid reason to mandate vitrification of US reprocessing waste. Chemical solidification to hydroceramic-type materials would be quicker, cheaper, simpler, and safer - especially with wastes that have already been calcined.
Second, hydroceramics possess a more fundamental advantage in that they already consist of the sorts of zeolitic "secondary phases" that glasses will eventually decompose to17,18 in likely repository scenarios. Hydroceramic materials would be especially stable relative to glass under the hydrothermal "worst case" conditions assumed by DOE's repository modelers (i.e., intrusion of liquid water into the burial region before the initial fission product heat pulse decays away). The zeolitic-tuff and aluvium at the NTS is typically rich in zeolites including clinoptilolite [K2,Na2,Ca(Al6Si30)O72 · 24H2O] and poorly crystalline kaolinite-like phases4,19. The thermodynamic stability of buried hydroceramic-type waste forms could be further enhanced by embedding them in-situ with a cheap grout "backfill" formulated to match what was put into the canisters.
Finally, it may not even be necessary to use autoclaves to make them. Any waste forms made from US-type reprocessing waste will have to be temporarily stored on site - probably for several decades - until decision-makers choose to implement an appropriate repository for this sort of radwaste4-6. During that time, hydroceramic grouts could be cheaply/safely cured at a temperature near that of boiling water by providing the interim storage facility with thermal insulation and simple space heaters.
ACKNOWLEDGEMENTS
We thank Greg Barger (Ashgrove) and Dick Evans (DiaSource) for providing us with samples of pozzolans. Partial support of DOE OBES and PETC is also acknowledged.
REFERENCES
FOOTNOTES
a
Reprint requests should be addressed to this author.b
In this paper "grout" refers both to the mix of inorganic materials to be cured into a concrete-like solid and to the finished product. It is also used as a verb to denote the process.c
Sugar calcination of this type of waste was implemented at INEEL three decades ago and then independently rediscovered by a Hanford subcontractor in 1995. The same process is implemented in rotary kilns at modern British & French reprocessing facilities.d
Porewater removal is a key element of the DUETAP process performed to prevent radiolytic pressurizatio of sealed canisters. In this case, the purpose was to reduce the degree of uncertainty regarding the exact composition of what was to be leach-tested.