PREPARATION OF PLUTONIUM ZIRCONATE AS AN INTERMEDIATE
STEP TO MAKING A HIGHLY DURABLE PLUTONIUM WASTE FORM
Thomas P. O'Holleran and Stephen G. Johnson
Argonne National Laboratory-West
P. O. Box 2528
Idaho Falls, ID 83403-2528
Peter C. Kong and Bruce A. Staples
Idaho National Engineering and Environmental Laboratory
P. O. Box 1625
Idaho Falls, ID 83415-2210
ABSTRACT
The Department of Energy selected immobilization for disposal in a geologic repository as one approach for disposing of excess plutonium. Materials for immobilizing weapons-grade plutonium for repository disposal must meet the "spent fuel standard" by providing a radiation field similar to spent fuel. Such a radiation field can be provided by incorporating fission products from high-level waste into the waste form. Recent experiments have demonstrated the feasibility of using high-level waste stored at the Idaho Chemical Processing Plant to prepare plutonium for repository disposal. Both glassy and crystalline materials were tested. Some of the crystalline materials were formed by devitrifying glasses prepared using conventional glass melting methods. During devitrification, plutonium was partitioned into crystalline host phases that proved highly durable, and resulted in higher plutonium loadings than could be achieved by dissolution in the initial glass. The most effective plutonium host phase identified in these experiments was a plutonium zirconate solid solution with a face-centered cubic (fluorite type) crystal structure. This material is easily prepared by direct solid state reaction of the oxides, and can accommodate appreciable quantities of rare earth neutron absorbers. Plutonium release rates were on the order of 10-5 to 10-6 g/m2/d as measured using the Product Consistency Test (PCT-A).
The ease with which plutonium zirconate can be prepared suggests a versatile alternative route to preparing plutonium from a variety of sources (weapons components, scrap, residues, etc.) for disposal. Plutonium converted to oxide can be immediately reacted with zirconia and rare earth neutron absorbers to form the solid solution. In this form, the plutonium is safer and easier to handle. Experiments have shown that the plutonium zirconate can subsequently be incorporated into a high-level waste glass so as to meet the spent fuel standard for repository disposal. Because of the chemical inertness of the zirconate solid solution, other materials and processes (e. g. cement, Synroc) could be used for consolidation, expanding the options for plutonium immobilization. Plutonium release rates are determined primarily by the highly durable zirconate host phase.
INTRODUCTION
In their report on dispositioning of excess weapons grade plutonium (1), the National Academy of Sciences recommended two options:
The second option is referred to as the "spent fuel standard." The Department of Energy (DOE) recently issued a Record of Decision (ROD) as the culmination of the process established by the National Environmental Policy Act (NEPA) selecting a hybrid approach using both the MOX fuel approach and the spent fuel standard for dispositioning of appropriate portions of the Nation's excess weapons-grade plutonium (2). The preferred approach uses a can-in-canister arrangement that expedites the schedule for dispositioning of weapons grade plutonium by using the operational HLW glass melter at the Defense Waste Processing Facility (DWPF) at the Savannah River Site. In the can-in-canister approach, plutonium is immobilized as the oxide in a special ceramic formulation using the Synroc process. The plutonium-bearing ceramic is encapsulated in stainless steel cans which are suspended inside a DWPF high-level waste glass canister. High-level waste glass is then poured into the canister, encapsulating the plutonium ceramic-containing cans.
The Idaho Chemical Processing Plant (ICPP) at the Idaho National Engineering and Environmental Laboratory (INEEL) reprocessed highly enriched reactor fuel, primarily from naval propulsion reactors, to recover the unburned highly enriched uranium. The reprocessing operation produced an aqueous HLW stream that was converted to a granular solid in a fluidized bed calciner to reduce volume. The solidified HLW calcine (approximately 3800 m3) (3) is currently stored at the ICPP in shielded underground facilities called binsets. Unlike other reprocessing operations, the operation at the ICPP did not chop the fuel to dissolve the fuel meat. Instead, the entire fuel element was dissolved. As a result, elements from the cladding are included in the HLW. Consequently, much of the calcine stored at the ICPP is rich in zirconium. A variety of processes and waste forms have been investigated as candidates for preparing the calcine for ultimate disposition in a geologic repository. As part of this effort, borosilicate glass formulations were developed that were tailored to the unique calcine chemistry (4,5).
The feasibility of using HLW stored at the ICPP to "denature" excess weapons grade plutonium to meet the spent fuel standard has been demonstrated (6). Initial efforts focused on vitrification technology, which has a proven track record world-wide for immobilizing HLW. It was recognized that the unique chemistry of the HLW at the ICPP, when combined with borosilicate glass, had the potential for yielding plutonium host phases that would not only be more chemically durable (and therefore proliferation resistant) than borosilicate glass, but that might also achieve overall plutonium loadings higher than solubility limits would permit in a conventional borosilicate glass. For example, it was initially proposed that plutonium might be partitioned into a known durable transuranic host like zircon (7-9) formed from zirconia in the HLW and silica in the glass-forming additives by devitrifying the glass. The plutonium-bearing zircon would be microencapsulated in a residual glass matrix containing fission products that provide the diversion-resistant radiation field to meet the spent fuel standard. Following the ROD, a modification to the initial approach was evaluated that could be implemented as part of the preferred approach selected by the DOE. The modified approach achieved the spent fuel standard using a two step process:
TECHNICAL APPROACH
Initially, experiments focused on devitrification of glass formulations developed in the late 1970s and early 1980s to immobilize zirconia-rich calcine in an attempt to produce durable crystalline plutonium host phases (10). Cerium was used as a surrogate for plutonium in these initial experiments, and surrogate (non-radioactive) calcine that was used during development of waste forms for HLW was substituted for the radioactive material. Samarium was added to simulate candidate neutron absorbers. Neutron absorbers would be added to materials destined for geologic disposal for criticality control. Verification experiments using plutonium were performed after promising formulations and processing conditions were identified with surrogates. A zirconia-based crystalline phase with a strong affinity for cerium, plutonium, and samarium and that appeared to form readily was identified in these experiments. This material was selected as the plutonium-bearing crystalline host phase for the two step HLW glass encapsulation approach. Synthesis conditions were first defined using cerium as a plutonium surrogate, then the plutonium zirconate was synthesized. Leaching tests were performed to confirm that the zirconate is a durable host phase for plutonium. Finally, a melting test was performed to demonstrate that the plutonium zirconate could be encapsulated in a HLW glass without significant alteration of either the plutonium zirconate or the glass matrix.
DEVITRIFICATION EXPERIMENTS
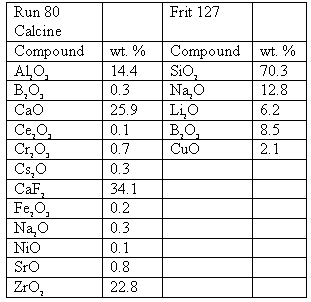
Devitrification experiments used a mixture of simulated zirconia calcine called Run 80 calcine and a glass additive called Frit 127 in a 3/7 ratio by weight to form the simulated HLW glass. The compositions of each of the starting materials is given in Table I. This formulation was originally developed to immobilize zirconia calcine (4). To this mixture was added 6 weight percent Sm2O3 and 9.5 weight percent CeO2 (equivalent to 15 weight percent PuO2 on a molar basis).
Table I. Compositions of Run 80 Calcine (non-radioactive simulant) and Frit 127 Glass.

The simulated HLW glass with CeO2 and Sm2O3 was melted at 1050°C in an alumina crucible using an electric resistance furnace. After a four hour hold at 1050° C, the furnace temperature was reduced to 500° C (at the furnace cooling rate), held for four hours, then raised to 700° C at 2° C per minute. The material was held at 700° C for 144 hours, then furnace cooled. The 500° C hold was intended to promote crystal nucleation, and the 700° C hold was to promote crystal growth (devitrification).
The resulting material consisted of three crystalline phases embedded in a glassy matrix. The primary cerium-containing phase was identified as a face centered cubic cerium zirconate (fluorite structure) with a cerium to zirconium ratio of 1.36/1 on a molar basis, and containing varying amounts of Sm2O3 up to about 10 mole percent. An anorthite-like sodium calcium aluminosilicate of somewhat variable composition also contained up to a few mole percent CeO2 and Sm2O3. No cerium or samarium was detected in either the other crystalline phase, an unidentified calcium silicate, or the glassy phase. The cerium zirconate phase was observed consistently in subsequent devitrification experiments, even when "seed" materials were added such as ZrSiO4 or TiO2 powder to promote the formation of other zirconate phases. Verification experiments with plutonium showed an even stronger tendency to form plutonium zirconate, as no plutonium was detected in the anorthite-like sodium calcium aluminosilicate phase.
PLUTONIUM ZIRCONATE SYNTHESIS AND CHARACTERIZATION
Actinide-bearing zirconias have been identified in the solidified material that formed after the Chernobyl accident, and have been suggested as durable actinide host phases (11)(9). Any plutonium host phase that is to be encapsulated in a HLW glass should resist dissolution in the aggressive silicate melt. Since a cubic zirconia phase heavily substituted with plutonium (or cerium) formed readily in many of the devitrification experiments (indicating thermodynamic stability in the silicate melt), an effort was initiated to synthesize the material in pure form.
Energy dispersive X-ray spectroscopy (EDXS) was used to acquire chemical data during scanning electron microscopic examinations of devitrified materials. Initially, cerium was used as a surrogate for plutonium to determine an effective firing schedule. An averaged CeO2/ZrO2 molar ratio of 1.36/1 was selected from these data. Materials were prepared both without Sm2O3 and with 8.5 mole percent Sm2O3. After a series of experiments, it was determined that cerium zirconate (both with and without Sm2O3) could be prepared simply by using reagent grade oxide powders, mechanically mixed and placed loose in an alumina crucible, and heated to 1675° C for 24 hours. This same procedure was used to prepare plutonium zirconate at a 1.36/1 PuO2/ZrO2 molar ratio (both with and without Sm2O3), except that a 48 hour soak at temperature was used to ensure complete reaction. Figure 1 shows X-ray diffraction patterns of plutonium zirconate both with and without Sm2O3, and an X-ray diffraction pattern of PuO2 for comparison. All three patterns index to the fluorite structure, and closely match patterns from devitrified glass samples. Lattice parameters for the plutonium zirconates are 0.528 nm without Sm2O3, and 0.533 nm with Sm2O3. Lattice parameters were calculated using P. E. Werner's TREOR90 algorithm. These data were used to calculate theoretical densities of 9.38 g/cm3 and 9.64 g/cm3 for the plutonium zirconate prepared without and with samaria respectively. "Extra" low intensity peaks in the zirconate patterns are attributed to small amounts of unreacted materials.
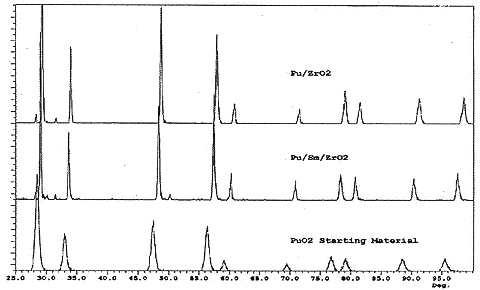
Fig. 1. X-ray diffraction patterns of plutonium zirconate phases, (top) without Sm2O3 (lattice parameter 0.528 nm), (middle) with Sm2O3 (lattice parameter 0.533 nm), and (bottom) pure PuO2 for reference (lattice parameter 0.540 nm). The ordinate is in arbitrary intensity units. Lattice parameters were calculated using P. E. Werner's TREOR90 algorithm.
Normalized release rates for plutonium from the plutonium zirconates with and without samarium were measured using the seven day Product Consistency Test (12). ASTM Type I water was used as the leachate (at a zirconate surface area to leachate volume ratio of 2000 m-1). Plutonium concentrations in the leachates were calculated from measurements of plutonium activity in samples of the leachate. Normalized plutonium release rates for the plutonium zirconate without Sm2O3 was 4.26 X 10-6 g/m2/d and for plutonium zirconate with samarium was 1.3 X 10-5 g/m2/d. These numbers are considered maximum release rates, as it is likely that the leachate samples picked up some plutonium contamination from the glove box environment as they were transferred from the leaching vessels. Nevertheless, these numbers compare favorably to what is considered an "acceptable" release rate of 1 g/m2/d for HLW glass. Leachate pH for the samarium-containing material was 7.58, and conductivity was 0.049 milli-Siemens/cm, compared to a pH of 6.03 and conductivity of 0.010 mS/cm for control blanks. The small differences in pH and conductivity between the samples and controls indicate very little release of material into solution. Unfortunately, insufficient leachate was available to perform these measurements on the plutonium zirconate leachate. These data indicate that plutonium zirconate is an extremely durable plutonium host phase, and demonstrate the feasibility of the first step of the proposed two step process for plutonium dispositioning (immobilization in a durable host phase, followed by encapsulation in a HLW glass).
PLUTONIUM ZIRCONATE ENCAPSULATION IN HLW GLASS
In order to establish the feasibility of the second step of the proposed two step process for plutonium dispositioning (encapsulation of the durable host phase in a HLW glass), it was necessary to show that neither the plutonium host phase nor the HLW glass matrix is altered by the encapsulation process in any way that would compromise the ability of the composite material to isolate radionuclides from the environment. Since both the plutonium zirconate and the HLW glass effectively isolate radionuclides from the environment, avoiding chemical reactions between the two during the encapsulation step presumably allows both components to retain their ability to isolate radionuclides from the environment in the resulting composite.
A sample of HLW glass was prepared by mixing Frit 127 and Run 80 calcine (see Table I) in a 7/3 weight ratio, and melting in an alumina crucible using an electric furnace. The glass was held at the melt temperature (1100° C) for four hours, cooled at the furnace cooling rate to 500° C for a 15 minute annealing hold, then furnace cooled to room temperature. Sufficient plutonium zirconate powder was spread evenly over the surface of the simulated HLW glass (still in the crucible) to give a 20 weight percent loading of plutonium based on glass mass. Figure 2 shows a scanning electron micrograph of a sample of the plutonium zirconate powder used for this experiment. The crucible was returned to the furnace and heated to 1050° C, held for four hours, cooled to 500° C for a 15 minute annealing hold, then cooled to room temperature. The crucible with the resulting HLW glass/plutonium zirconate composite was sectioned and polished for scanning electron microscope (SEM) examination.
The dense plutonium zirconate had settled to form a glass/plutonium zirconate layer about half the depth of the melt with a well defined boundary layer. This boundary layer is shown in Figure 3a. The light phase in Figure 3a is plutonium zirconate, and the dark phase is the simulated HLW glass as identified by EDXS. No plutonium or other variation in composition could be detected in the glassy phase, either above or within the composite layer. The volume ratio of HLW glass to plutonium zirconate in the composite layer was estimated at about 0.5, which would give an average plutonium concentration in the composite layer of about 43 weight percent. This gravity-driven concentration of plutonium shows that plutonium loadings in excess of the targeted 20 weight percent can be easily achieved.
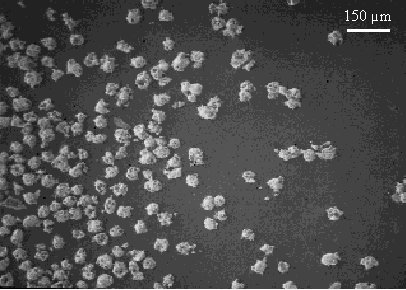
Fig. 2. Secondary electron image of a sample of the plutonium zirconate powder used in the HLW glass encapsulation experiment. The magnification in this figure is the same as in Figure 3.
Zircon grains from the crucible were incorporated into the melt near the glass/crucible interaction zone. Zircon grains appear as the medium gray phase in Figure 3b. Note that no zircon grains are visible in the glass phase in Figure 3a, and, while close examination will reveal two zircon grains in the glass/plutonium zirconate layer in Figure 3a (near the bottom, right of center), zircon grains were very rare except near the crucible wall. These observations, together with the fact that zircon crystals have never been observed in this glass, even after devitrification, strongly suggests that the zircon grains entered the melt via a crucible erosion mechanism. EDXS analysis of zircon grains incorporated into the glass melt showed that these grains had absorbed about 3 to 4 mole percent PuO2, while zircon grains in the crucible showed no detectable plutonium. EDXS analysis of matrix glass phase in the immediate vicinity of zircon and/or plutonium zirconate phases near the interaction zone showed no detectable plutonium in the glass phase.
A likely explanation for this observation is that plutonium is sufficiently soluble in the glass at the melt temperature (1050° C) to allow diffusion from the plutonium zirconate phase into zircon grains from the crucible. If there is any residual plutonium in the glass in excess of about 0.5 atom percent (estimated detection limit), as the glass cools and plutonium solubility decreases it most likely partitions into the more thermodynamically stable zirconium-containing crystalline phases. Had zircon not been introduced into the melt from the crucible, any plutonium dissolved into the glass from the plutonium zirconate at elevated temperature (in excess of about 0.5 atom percent) would have returned to the zirconate phase during cooling, leaving both materials essentially unaltered. In either case, the net amount of plutonium transported to the zircon grains was small, resulting in no detectable changes in either the plutonium zirconate or the simulated HLW glass, thus demonstrating the feasibility of encapsulating plutonium zirconate in a HLW glass.
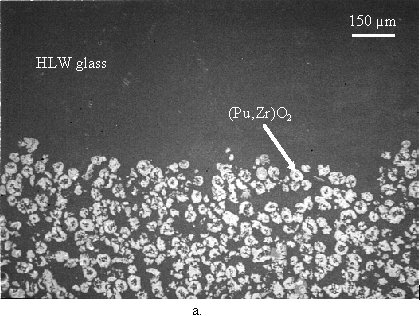
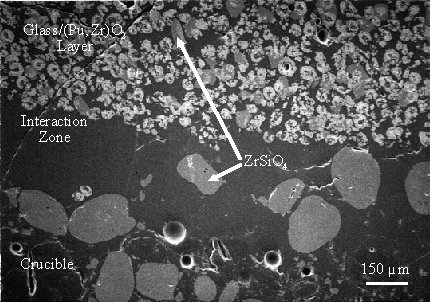
Fig. 3. Secondary electron micrographs of a. the boundary between the simulated HLW glass (dark phase) and the glass/plutonium zirconate composite layer, and b. the glass/crucible interaction zone showing zircon grains from the crucible incorporated into the glass/plutonium zirconate composite layer.
CONCLUSIONS
First identified in devitrification experiments, plutonium zirconate was synthesized and shown to be a highly durable crystalline plutonium host phase. In the context of a two step process that first immobilizes plutonium then encapsulates the immobilized product in a HLW glass, plutonium zirconate meets the criteria for the product from the first step. The ease with which plutonium zirconate can be synthesized also suggests that the first step could be a fairly simple process. The second step in the process, encapsulating plutonium zirconate in a HLW glass, has also been demonstrated by mixing the plutonium zirconate with a simulated HLW glass at high temperature without discernibly altering either phase. The two step process could be implemented either by adding plutonium zirconate directly to the melt in a HLW glass melter, or by adding plutonium zirconate to the canister during glass pouring. The plutonium zirconate could either be added directly to the canister, or added to the glass stream using an intermediate heated funnel arrangement. Several process related requirements can be derived from these experiments:
If the final product is poured into a DWPF size canister, meeting this requirement by natural cooling will likely be assured given the thermal characteristics of the material and the geometry of the canister.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of M. Hansen, B. Scholes, B. Boyle, T. Morris, M. Hankins, S. Frank, E. Wood, and M. Meyer. Argonne National Laboratory is operated for the U. S. Department of Energy by the University of Chicago. This work was supported by the U. S. Department of Energy, Reactor Systems, Development and Technology, undedr contract W-31-109-ENG-38.
REFERENCES