GARNET SOLID SOLUTION OF Y3AL5O12-GD3GA5O12-Y3GA5O12
(YAGGGGYGG) AS A PROSPECTIVE CRYSTALLINE HOST-PHASE
FOR PU IMMOBILIZATION IN THE PRESENCE OF GA
B. E. Burakov, E. E. Strykanova
V. G. Khlopin Radium Institute
28, 2nd-Murinskiy Ave., St. Petersburg, 194021, Russia
Tel: +7+812 346-1186, Fax: +7+812 346-1129, E-mail: burakov@riand.spb.su
ABSTRACT
Weapons plutonium pits contain a significant admixture of Ga (up to 3 wt.%) in the form of a metallic alloy with Pu. The different chemical behavior of Ga in comparison with Pu can impact the process for treating weapons grade plutonium to produce a durable waste form. As was shown in previous work, yttrium-aluminum garnet (YAG) Y3Al5O12 is a very durable crystalline host-phase with a lattice of high capacity for incorporating actinides. Crystalline ceramics based on YAG have been proposed for actinide transmutation and as components of Pu-containing nuclear fuel. This paper considers a solid solution of YAG with other garnets: Gd3Ga5O12 (GGG) and Y3Ga5O12 (YGG) as a prospective host-phase for immobilization of weapons grade Pu in the presence of Ga. The advantages are:
The physico-chemical features of a YAGGGGYGG solid solution are described. As a conclusion the optimal methods of YAGGGGYGG synthesis using existing technologies are discussed.
INTRODUCTION
Weapons plutonium pits contain a significant admixture of Ga (up to 3 wt.%) in the form of a metallic alloy with Pu. Also, some Pu-containing wastes have Ga admixture up to 8 wt.%. The different chemical behavior of Ga in comparison with Pu can impact the process for treating weapons grade plutonium to produce a durable waste form. The crystalline host-phases usually considered for Pu immobilization: zircon, monazite, zirconolite, etc. do not contain Ga in their lattices. There is also no information concerning Ga incorporation by these minerals in the form of solid solution. We suggest the system of crystalline garnet: Y3Al5O12 (YAG) –Gd3Ga5O12 (GGG)–Y3Ga5O12 (YGG) as a prospective host-phase for immobilization of weapons grade Pu in the presence of Ga.
Synthetic garnet with a general formula A3B2C3O12 where A, B and C are three types of structural cation positions, is a well-known durable compound widely used in industry such Nd-doped YAG1,2 as in the field of laser materials.3 Garnet is also desirable in the microelectronics industry as substrate for magnetic films with cylinder magnetic domains4 and as an artificial gem stone with a hardness higher than quartz and without cleavage problems.5,6 YAG was proposed previously as a host-phase for actinide immobilization.7-10
Garnet provides a high lattice capacity for incorporation of various chemical elements because of its flexible structure as described in the following:
The same elements can occupy different positions; for example, Al – "B" and "C", Gd – "A" and "B", Ga – "B" and "C". However, this does not decrease the stability of garnet crystalline structure.
Usually synthetic garnet is used in a form of high quality monocrystals, which are produced by melting methods11-13 at temperature from 1770oC (melting point of YGG) to 1950oC (melting point of YAG). However, garnet can be formed at significantly lower temperature (900oC for YAG) by solid-phase synthesis.14
In our experiments of synthesis of garnet solid solution YAGGGGYGG, we consider the possible application of cold-crucible melting techniques, which are widely considered as an attractive method for high level radioactive waste (HLW) treatment.15
EXPERIMENTAL
A combined nitrate solution of Ce and Ga (Ce/Ga ratio was 99/1) simulating weapons Pu pits dissolved in nitric acid was mixed with nitrate solutions of Y, Al, Gd. The concentrations of all elements in mixed solution was controlled to provide the stoichiometry of the final garnet YAGGGGYGG with the following formula (Y, Ce, Gd)3 (Al, Ga)5 O12 containing about 8 wt.% Ce and 0.6-0.7 wt.% Ga. The hydroxides of Y, Al, Ce, Gd, Ga were co-precipitated by adding excess of NH4OH. Dried hydroxide powder was sintered at a temperature 800oC for 30 minutes. The resulting product was milled and cold pressed to form pellets 8 millimeters in diameter and 20 mm in height. Pellets were heated in air for 20-30 seconds under a temperature gradient ranging from 1500°C in the bottom part to 2000°C in the top part of the pellet. Heating was stopped when melting of the top part of the pellet was visible. Samples of the sintered ceramic were examined by X-ray diffraction, and the melted part of the samples was also examined by SEM.
RESULTS AND DISCUSSION
XRD-analysis of synthesized samples show that the yield of garnet (Y,Ce,Gd)3(Al,Ga)5O12 varied from 80 wt.% in the unmelted part to 87 wt.% in the melted part. The content of the other Ce-doped phase (Y,Gd)AlO3 with a perovskite structure is correlated inversely with the content of separated cerium phases: Ce2Y2O7 and CeO2. The unmelted part of the samples contains up to 13 wt.% of those separated Ce-phases, while the melted part contains significantly less – about 1-2 wt.%. At the same time a content of perovskite phase does not exceed 4 wt.% in the unmelted part but reaches 10-12 wt.% in the melted part. Cell parameters of the perovskite phase are significantly higher in the melted part compared with the unmelted part. Therefore, the formation of Ce-doped perovskite during melting is correlated to Ce assimilation from the initial separated Ce phases. This is also confirmed by the high Ce content of 11 to 26 wt.% in the perovskite from melted samples.
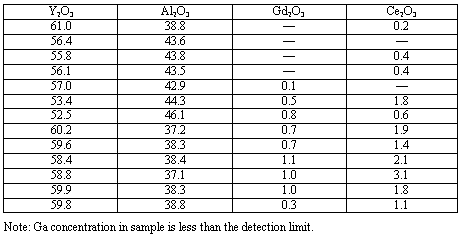
Microprobe analysis shows that the distribution of Ce and Gd in the garnet matrix is not uniform (Table I). Generally, the highest concentration of Ce is correlated with the highest concentration of Gd. The melted part of the samples is characterized by two main crystalline phases responsible for Ce-incorporation in their lattices: garnet (Y,Gd,Ce)3(Al,Ga)5O12 and perovskite (Y,Ce,Gd)AlO3 (Figure 1).
Table I. Chemical Composition of Synthesized Garnet (in wt.%) from
Microprobe Analysis (Normalized to 100%).

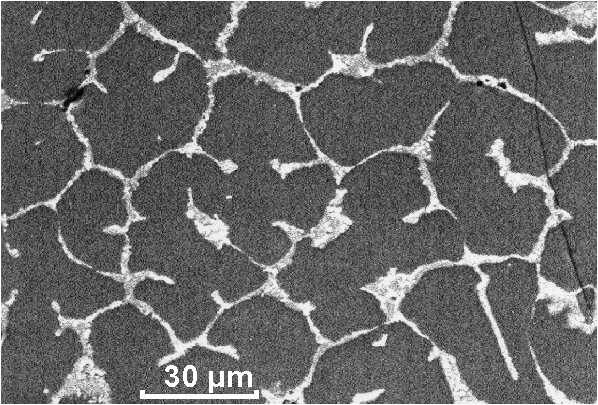
Figure 1. SEM back-scattered image of crystalline ceramic including: (a) dark – Ce-doped garnet phase and (b) light – Ce-doped YAlO3 of perovskite-type structure.
It is important to note that Pu valence behavior is not equivalent to Ce; and, in Pu experiments, we can expect other Pu distributions between garnet and perovskite phases, even the formation of a garnet mono-phase. In case of actinide incorporation in a tetravalent state, the necessity of using bivalent elements for charge compensation is proposed: (Y,Gd,Pu,Np,Me)3(Al,Ga)5O12, where Me is a bivalent element.
CONCLUSIONS
The results allow us to make the following preliminary conclusions:
The proposed schedule of Pu conversion into garnet-based ceramic is shown on Figure 2.
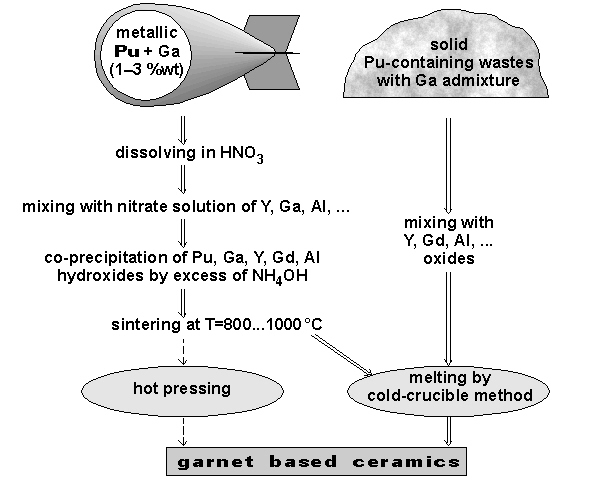
Fig. 2. Proposed schedule of Pu conversion into garnet-based ceramic.
ACKNOWLEDGMENTS
The authors are very grateful to Mrs. M. Zamoryanskaya and Mr. V. Garbuzov for their help. The authors thank Dr. D. Knecht for the comments and corrections.
REFERENCES
BACK