SUMMARY OF HLW GLASS-CERAMIC WASTE FORMS
DEVELOPED IN IDAHO FOR IMMOBILIZING PLUTONIUM
Dieter A. Knecht, Krishna Vinjamuri, Swami V. Raman, Bruce A. Staples,
and Jon D. Grandy
LMITCO
Stephen Johnson, Thomas P. O'Holleran, and Steven Frank
ANL-W
ABSTRACT
New glass-ceramic forms have been developed that may be suitable for immobilizing plutonium, based on tests using plutonium and cerium as a surrogate for plutonium. The glass-ceramic waste forms were formed with plutonium oxide loadings of 15-17 wt.% and cerium oxide loadings equivalent to 15 wt.% of plutonium oxide. All samples were formed with surrogate compositions of Idaho Chemical Processing Plant (ICPP) high-level waste (HLW) calcines either by melting at 1450oC, sintering at 1050oC and 138 MPa in a hot isostatic press (HIP), or by cold-pressing at 34.5 MPa followed by atmospheric constant-rate or isothermal sintering at 1050-1250oC. Calculations show that if radioactive calcine were used, these materials would result in a dose considered by DOE Order 5633.3B to be self-protecting and greater than 200 rem at 1 m, assuming the dimensions of a HLW glass canister. X-ray diffraction and scanning electron microscopy (SEM) showed that durable host crystalline phases for plutonium or cerium were formed, including fluorite, zircon, zirconolite, zirconia, and alumina. Durability tests using the Product Consistency Test (PCT) at 90oC and 7 days at a surface-to-volume ratio of 500 m-1 resulted in a Pu release of 0.001 g/m2 and Ce releases less than 0.001 g/m2. Other components were detected at comparable levels to Hanford standard ARM-1 glass and at levels of 20-200 times lower than for the Savannah River Site standard EA glass.
INTRODUCTION
The shutdown of the weapons production left a wide variety of plutonium-containing scrap and residue materials in the manufacturing "pipeline." A report by the National Academy of Sciences (NAS)1 presented two alternatives for disposition of residual plutonium by (1) using fuel in existing or modified reactors and (2) vitrification in a mixture with radioactive waste. The Record of Decision for Storage and Disposition of Weapons-Usable Fissile Materials Final Programmatic Environmental Impact Statement2 selected these two options for plutonium disposition including immobilizing of Pu in a mixture with radioactive wastes as a glass or ceramic. Some of the waste forms that have been suggested or shown to be good candidates for immobilizing plutonium include crystalline zircon, zirconolite and zirconia ceramics and glass ceramics consisting of a durable glass phase embedded with some of these ceramic phases.3, 4, 5, 6
The Idaho Chemical Processing Plant (ICPP), which is a part of the Idaho National Engineering and Environmental Laboratory (INEEL), has stored and reprocessed irradiated nuclear fuel from 1953-1992 to recover uranium-235 and krypton-85 for the U.S. Department of Energy (DOE). The high-level liquid radioactive waste (HLLW) has been solidified to a granular calcined waste, which is stored in near-surface, stainless-steel bins inside concrete vaults.7 Glass-ceramic waste forms have been developed for ICPP high-level wastes (HLW).8,9,10 Detailed microstructure analysis of these waste forms revealed the presence of actinide host phases, including fluorite, stabilized cubic zirconia, zircon, zirconolite, perovskite, and amorphous aluminosilicate glass.8, 9,10
This paper summarizes glass-ceramic materials developed at Idaho for immobilizing plutonium. Glass-ceramic waste forms have been prepared using ICPP calcine surrogate compositions, plutonium or cerium as a surrogate for plutonium, samarium as a neutron absorber, and other additives. The glass ceramics were formed in a crucible melt, hot isostatic press (HIP), or by cold pressing and sintering.
ICPP CALCINE CHARACTERISTICS
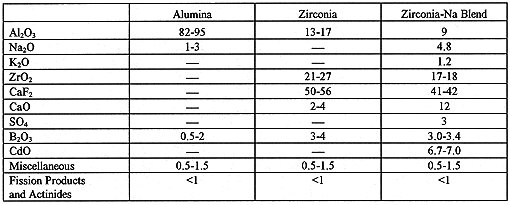
Currently there is an inventory of 3,800 m3 HLW calcine at ICPP consisting primarily of alumina and zirconia-based calcines,11 resulting from the dissolution of aluminum and zirconium fuels, respectively. Ziconia-sodium blended calcines resulted from waste minimization efforts to blend a non-HLW sodium decontamination waste with zirconia HLLW. Table I shows the ranges of compositions of the different calcines. The current inventories of alumina, zirconia and zirconia-sodium calcines are approximately 560, 1250, and 1750 m3, respectively. An additional 240 m3 calcine inventory consists of calcines from processing other minor fuel types and start-up bed materials. Fission product content is typically less than 1 wt.% but results in total radioactivity of 170 and 70 TBq/m3 (47,000 and 19,000 Ci/m3) in alumina and zirconia calcine, respectively. Microshield code calculations predicted that immobilized plutonium in glass-ceramic waste forms containing 22 to 60 wt.% of radioactive calcine would exhibit radiation dose rates12 of 100 to 600 rem/hr at 1 m, which would be considered to be self-protecting for nuclear materials according to DOE Order 5633.3B.
Table I. Types of Calcine at ICPP with Compositions in Wt.%.

BASIS AND APPROACH
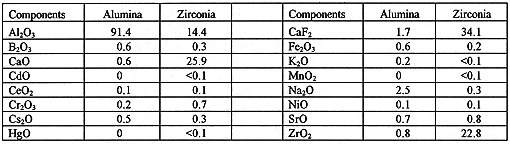
Glass and glass-ceramic waste forms for HLW disposal have been prepared previously from ICPP calcine compositions with glass and crystalline phases including zirconia, zirconolite and zircon, which have also been proposed as a waste form for excess plutonium. These waste forms have exhibited high durability as measured by standard leach tests comparable to other HLW glasses. Similar HLW waste forms prepared from ICPP calcine and containing plutonium were calculated to have a high radiation dose and thus provide additional protection against diversion if used to immobilize plutonium. Because of these results, laboratory tests were run to immobilize plutonium or cerium as a surrogate for plutonium.13 The approach consisted of mixing the surrogate calcine, additives, samarium oxide as a neutron absorber, and cerium or plutonium oxide and forming a ceramic material by one of three methods: (1) melting at 1450oC with 50-60 wt.% calcine, (2) hot isostatic pressing at 1050oC and 138 MPa (20,000 psi) with 50-60 wt.% calcine, or (3) cold pressing at 34.5 MPa (5,000 psi) and sintering at 1050-1250oC with 30-50 wt.% calcine. Waste forms were characterized for density, chemical durability (leachability), and microstructure by X-ray diffraction (XRD) and scanning electron microscopy (SEM). SEM with associated energy-dispersive X-ray fluorescence spectrometry (EDS) was used to identify predominant crystalline and amorphous phases in the glass-ceramic samples. Table II shows the compositions of surrogate alumina and zirconia calcines used in these tests.
Table II. Compositions of Surrogate Zirconia and Alumina Calcines (wt.%).
ZIRCONIA CALCINE RESULTS
The composition of surrogate zirconia calcine used in these tests is shown in Table II. The glass-ceramic formation conditions are shown in Table III. The tests described here include a HIP test (GC-71) and a melt test (CWF-1) using Ce as Pu surrogate13,14 and one melt test (CWF-1Pu) using Pu.15 Figure 1 is an optical transmission micrograph of CWF-1 at X50 magnification showing zirconolite rods (CaZrTi2O7), alumina laths, and a Ca-Al-Si glass. The cerium as a plutonium surrogate was found primarily in the zirconolite structure, which is desired for long-term disposal. In a previous study, Ce was also found in zircon.10 In other HIP ceramic experiments using different calcine composition, zirconia and Ca-Al-Si glass are also formed, but sphene is present instead of zirconolite, with Ce present mainly in the zirconia and sphene phases, also desired for high durability.14 Figure 2A is a SEM image at X2000 magnification of a ceramic sample GC-71 formed in a HIP at 138 MPa and 1050oC and identifies a large orthorhombic, forsterite-type crystal morphology with analyzed atomic ratios consistent with the formula, Ce0.48Ca0.48Sm0.56Zr0.20Si1.0O4-x, small zirconia (ZrO2) crystals and a Ca-Al-Si glass phase. This structure was originally reported14 as a zircon-type morphology, but the cation charge and SEM-EDS analysis appear to be more consistent with the current forsterite morphology. The sample CWF-1 was formed by melting at a higher temperature of 1450oC compared to sample GC-71 formed at a lower temperature and high pressure in a HIP. The cerium is present mainly in the zirconolite phase, which is desired for high durability. Sample CWF-1Pu was produced at the same temperature and compositions of calcine and additives as CWF-1 but substituting plutonium on a molar basis for cerium. Figure 2B shows the SEM at a X500 magnification also with zirconolite and zirconia as primary crystalline phases in a Ca-Al-Si glass. The plutonium was present mainly in the zirconia phase as a (ZrxPu1-x)O2 mixture, which is a desired plutonium host phase. X-ray diffraction results for these materials confirm the above crystalline phases as well as calcium fluoride (fluorite, CaF2) present at the largest quantities. This crystal is also visible in the SEM picture in Figure 2. The glass phases in samples GC-71 and CWF-1 appeared to contain about one mole percent Ce.
Table III. Compositions (wt.%) for Pu/Ce Immobilization Tests using
Surrogate Zirconia Calcine.

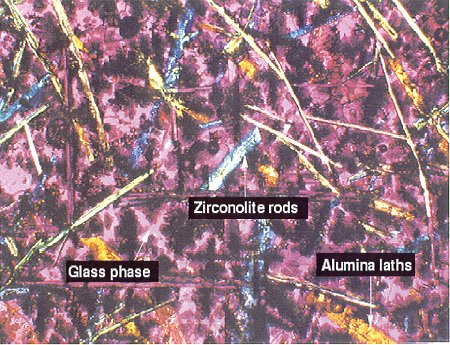
Fig. 1. Transmission, cross-polarized optical microscopy image of glass-ceramic sample CWF-1 formed in a high-temperature melt.
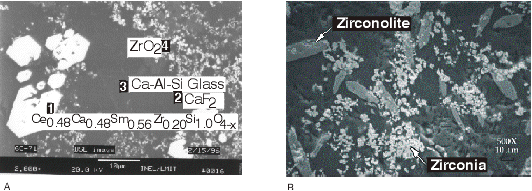
Fig. 2. (A) SEM backscattered electron image of glass-ceramic sample GC-71 formed in a HIP using Ce as a Pu surrogate. (B) SEM backscattered electron image of glass-ceramic sample CWF-1Pu formed in a high-temperature melt using Pu.
The durability was measured using the 7-day PCT at 90oC at a surface to volume ratio of about 500 m-1, and the results are summarized in Table IV. In all cases, the normalized elemental releases for Ce and Sm were below detectability of 0.05 g/mL and 0.02 g/mL, respectively. The other components were comparable to the Hanford standard ARM-1 glass, indicating a high durability. The leach rates were much lower than for the Savannah River Site standard EA glass when run under the same surface-to-volume conditions.
Table IV. Normalized 7-day PCT Elemental Release (g/m2) for
Melted, HIPed
and Sintered Glass-Ceramics.a

ALUMINA CALCINE RESULTS
The composition of surrogate alumina calcine used in these tests is shown in Table II. The glass-ceramic formulation conditions are shown in Table V. The tests include a HIP test, GC-81; two sinter tests, GC-82 and GC-83, using Ce as a Pu surrogate; and two sinter tests, GC-82Pu and GC-83Pu, using Pu.13 The purpose of the HIP test at 1050oC and 130 MPa was to provide a "baseline" material with cerium as a surrogate for the sinter tests, because these HIP conditions are most conducive for full reaction and densification of the components. The sinter tests involved cold pressing pellets at 34.5 MPa (5,000 psi) followed by atmospheric constant-rate or isothermal sintering at 1050-1450oC for 1-30 hr. A similar sintering process has been used commercially to form nuclear fuel pellets, including MOX fuel and (PuZr)O2 fuel16 pellets. The purpose of these sintering tests was to determine if this process would form similar durable phases as were formed during HIPing and if sintering is a feasible option for waste forms prepared with ICPP calcine compositions.
Table V. Compositions (wt.%) for Pu/Ce Immobilization Tests Using
Surrogate Alumina Calcine.
Figure 3 shows a SEM micrograph image magnified X610 of the sample GC-81 prepared by HIPing and contains cerium-samarium oxide, alumina, ZrO2, TiO2, and an aluminosilicate glass phase. Figure 4 shows SEM micrograph images for sintering tests with Ce or Pu and with two different formulations as shown in Table V. Sample GC-82 (Figure 4A) was sintered at 1450oC for 30 hr using Ce as a Pu surrogate. Spot 1 represents a cerium-samarium oxide with atomic ratio Ce:Sm of 45:3. Sample GC-82Pu (Figure 4B) was also sintered at 1450oC for 30 hr but using Pu with otherwise the same calcine and additive components as GC-82. The Pu-Sm oxide shown in spot 1 has atomic ratio Pu:Sm of 78:22. Sample GC-83 (Figure 4C) was sintered at 1400oC for 3 hr using Ce as a Pu surrogate. The cerium appears in spot 1 with atomic ratios Zr:Ce:Sm of 28:17:6. Sample GC-83Pu (Figure 4D) was sintered at 1450oC for 30 hr but using Pu with otherwise the same calcine and additive components as GC-83. The Pu mostly appears in the broader white circular morphology in the atomic ratio Zr:Sm:Pu of 54:14:32. The sintered samples, GC-82 and GC-83, showed volume shrinkage around 40%, as would be expected. Crystalline phases present in sample GC-81 included ceria (CeO2), alumina, zirconia, mullite, and quartz, as determined by XRD. Figure 3 shows the SEM image at X610 magnification for GC-81, including zirconia, ceria phases and an aluminosilicate glass phase. In GC-82, ceria, alumina and aluminosilicate glass were detected by XRD and SEM, with the Ce present primarily in a ceria-type phase (see Figure 4A). The same formulation used with Pu in GC-82Pu resulted in the SEM image at X250 magnification in Figure 4B, and showed that Pu was present mainly in a plutonium-samarium oxide with a surrounding aluminosilicate glass phase. In GC-83, a zirconium-cerium-samarium oxide phase was formed containing most of the Ce as well as alumina and aluminosilicate glass (see Figure 4C). For the Pu sample, GC-83Pu, most of the Pu is in a zirconium-plutonium-samarium oxide, including alumina and aluminosilicate glass as in GC-83.

Fig. 3. SEM backscattered electron image of glass-ceramic sample GC-81
formed in a HIP.
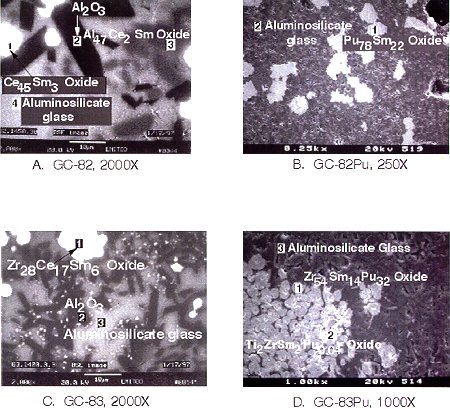
Fig. 4. SEM backscattered electron image of glass-ceramic samples formed by cold pressing and sintering: (A) Sample GC-82 sintered at 1450oC for 30 hr; (B) Sample GC-82Pu sintered at 1450oC for 30 hr; (C) Sample GC-83 sintered at 1400oC for 3.3 hr; and (D) Sample GC-83Pu sintered at 1400oC for 30 hr.
For the Ce-containing samples, the durability was measured using the 7-day PCT at 90oC at a surface- to-volume ratio of about 500 m-1. The results are summarized in Table IV for samples of GC-81, -82, and -83. Sample GC-82 was sintered at 1450oC and 10 hr, rather than 30 hr, for the samples used for the SEM in Figure 4A. Sample GC-83 was sintered at 1400oC for 10 hr, rather than 3.3 hr, for the sample used for the SEM in Figure 4C. Durability tests were not run for the Pu samples. In all cases, the normalized elemental releases for Ce and Sm were very low to below detectability of 0.05 g/mL and 0.02 g/mL, respectively. The other components were comparable to the Hanford standard ARM-1 glass, indicating a high durability. The leach rates were much lower than for the Savannah River Site standard EA glass when run under the same surface-to-volume conditions.
Thus, the tests with alumina calcine showed similar results for samples that were HIPed and sintered with similar behavior of Pu and Ce.
CONCLUSIONS
Based on these and other high-temperature melting studies, glass-ceramic compositions have been formed with zirconia and alumina surrogate calcines with highly durable glass and crystalline phases for hosting the fission products and plutonium or cerium as a plutonium surrogate. In the zirconia calcine glass-ceramic produced by devitrifying a high-temperature glass formed at 1400oC, the predominant phases hosting plutonium and neutron absorber samarium were zirconia and zirconolite. Similar results were observed using cerium as a plutonium surrogate by high-temperature melting at 1450oC or in a HIP at 1050oC and 138 MPa. Maximum cerium oxide loadings were 7 wt.% (plutonium oxide equivalent of 12 wt.%) in the melting experiments and 9 wt.% (plutonium oxide equivalent of 15 wt.%) in HIP experiments. In the HIP experiments, a forsterite-like phase was formed in addition to zirconia, sphene, and zirconolite as cerium and samarium host phases. Leach rates were low, in the range observed for the most durable HLW glasses at other sites, including the ARM-1 glass composition from Hanford.
A new approach to produce durable glass-ceramic forms by cold pressing followed by high temperature sintering produced highly durable materials as hosts for fission products cesium and strontium as well as for plutonium or cerium and samarium. This method using preliminary testing with alumina calcine and silicate-based frits resulted in major crystalline phases of corundum and ceria. Where zirconia and titania were added, a cerium oxide-zirconium oxide were also formed. Leach rates were similar to those observed for the most durable HLW glasses at other sites, including the ARM-1 glass composition from Hanford. Only a limited number of tests were run to determine PCT leach rates, crystal structure using XRD, and microscopic composition and phase structure using SEM. Thus additional samples that are available should be tested to complete the sintering experimental design matrix and the resulting scientific basis for determining the optimal conditions.
Since the cold press-sinter method is a mature commercial technology in use to prepare uranium oxide fuel and mixed uranium-plutonium oxide fuel pellets, it appears promising for future waste form application, including with zirconia-based calcines and high activity waste (HAW) or separated long-lived nuclear waste components including the actinides. The cold press-sinter process is also being developed at Lawrence Livermore National Laboratory (LLNL) for immobilizing plutonium in Synroc but could be used with the calcine and frit compositions from this study instead of Synroc. Thus, this technology could become a commercially viable option in the future.
Thus, a number of candidate glass-ceramic materials capable of immobilizing plutonium were prepared using plutonium or cerium as a surrogate for plutonium and surrogate zirconia-based ICPP calcines. The waste products have a high capacity for plutonium or cerium, up to an equivalent plutonium oxide loading of 17 wt.% while incorporating the neutron poison, samarium, in the same phases. The samples have a high durability based on the PCT and would use the radionuclides in the existing HLW to provide a spent fuel standard as a deterrence from theft. The processes can be run either by melting at 1450oC, HIPing at 1050oC and 138 Mpa, or by cold pressing at 34.5 MPa and sintering at 1050-1450oC and 1-30 hr.
ACKNOWLEDGMENTS
This work is sponsored by the U. S. Department of Energy, Idaho Operations Office, under contract DE-AC07-94ID13223. The authors would like to thank Bill Robson and Doug Wenzel for calculating the anticipated doses at 1 m of immobilized ICPP HLW.
REFERENCES