A SOURCE-TERM MODEL FOR CARBON-14 RELEASES
FROM AN ABOVE GRADE, CONCRETE, LOW-LEVEL
RADIOACTIVE WASTE DISPOSAL FACILITY
A.N. Findikakis, N.K. Copty, K.C. Kuo, M.A. Sabbe and R.F. Schulman
Bechtel National Inc.
ABSTRACT
This paper describes a methodology developed for the definition of the source term for above grade, earth-mounded, reinforced concrete low level waste (LLW) disposal facilities. The methodology is based on geochemical modeling using the geochemical computer code PHREEQC, and by accounting for the site-specific chemical conditions at the facility (equilibrium, pH, redox) and uses the. The paper focuses on the application of the method to the estimation of the carbon-14 source term. The model simulates the evolution in time of the chemical environment in concrete-bunker disposal cells with concrete-grout backfill, accounting for the interaction between infiltrating water and the cement grout and concrete of the facility, and estimates the solubility of carbon over time. The simulated conditions in the facility are compared with the three physicochemical environments used in the literature to describe the evolution of cement pore water chemistry, and often indicated as Environments I, II and III.
A sensitivity analysis was conducted to assess the significance of different assumptions used in the model. The sensitivity analysis focused on the following parameters: availability of carbon dioxide in the air-filled pore space of the waste, the pH of the water entering through the concrete roof slab of the vault, the availability of oxygen inside the concrete cells. The sensitivity analysis suggested that the pH of the leachate and the concentration of total carbon in the concrete are not sensitive to any of these parameters. However the characteristics of the infiltrating water do affect the pe of the leachate leaving the floor slab.
The model accounts for the effect on C-14 concentrations in the leachate of the constantly increasing available C-12 due to the generation of carbon dioxide as a result of the biodegradation of organic matter in the waste. The continuous generation of carbon dioxide (12CO2) and the formation and deposition of calcite (Ca12CO3) within the waste and concrete means that the ratio of C-12 and C-14 will continuously increase. The paper discusses how through isotopic exchange between the C-14 in the clacite and the increasing availability of C-12, the fraction of C-14 to C-12 in the solid as well as in the aqueous phases will decrease with time.
The application of the model suggests that in the analyzed facility Environment I conditions will exist in the pore water of the bottom slab during a relatively short period (of the order of 100 to 150 years), and Environment II conditions will persist during the rest of the 10,000-year time-frame of the performance assessment analysis. The pH of the pore water in the bottom slab of the CIC facility will remain higher than 12.6 during the 10,000 year time-frame of the performance assessment analysis.
INTRODUCTION
A critical element of the performance assessment of low level waste (LLW) facilities is the definition of the source term, i.e. the rate of nuclide release from the waste disposal units. To quantify this term for above grade, earth-mounded, reinforced concrete disposal LLW facilities, a methodology based on geochemical modeling and flow analysis was developed. The methodology accounts for the site-specific chemical conditions at the facility (equilibrium, pH, redox) and uses the geochemical code PHREEQC Version 1.3 developed by the U.S. Geological Survey (Parkhurst, 1995). This program can perform speciation, reaction-path, advective transport and inverse geochemical calculations.
This paper describes the application of the geochemical modeling approach to the estimation of the carbon-14 source term. The model simulates the evolution of the geochemical conditions within the facility as a function of time by accounting for the interaction of the infiltrating water with the cementitious environment surrounding the waste. This paper also provides a comparison of the simulated chemical conditions with the three-physiochemical environments used in the literature to describe the evolution of cement water chemistry, often indicated as Environment I, II and III. A detailed discussion of these three environments can be found in Krupka and Serne (1997).
CONCEPTUAL MODEL FOR AN ABOVE GRADE CIC CONCRETE
LLW FACILITY
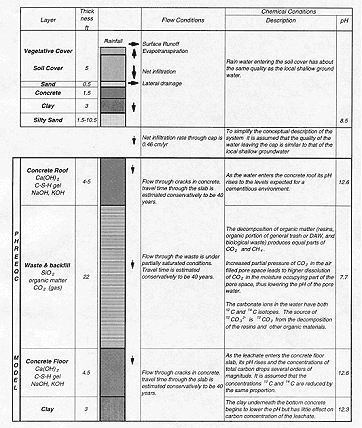
Changes in the quality of the water infiltrating through the capped disposal facility causing the generation of leachate are described by considering a column representing the different material layers in the disposal facility: the concrete roof, the waste, the concrete floor slab and an underlying clay layer. Figure 1 illustrates the key flow processes and chemical conditions through a typical section of an above grade concrete LLW disposal facility. Changes in the quality of infiltrating water are described by considering a column representing the different material layers in the facility. The model consists of four compartments: the concrete roof, the waste, the concrete floor slab and the underlying clay layer. To simplify the model, the soil cover above the concrete roof slab is not included. Rain water infiltrating through the soil cover reaches equilibrium. It is assumed that the quality of the water passing through the cap, which consists of native soils, will be similar to that of the local shallow ground water system.
A significant part of the low-level radioactive waste, including the dry activated waste (DAW), resins and the biological waste, consists of non-radioactive organic compounds, which are expected to biodegrade. The biodegradation rate of different organic compounds will vary. Simpler compounds will degrade faster then more complex compounds such as the resins. It is assumed that the organic matter in the waste will biodegrade within 200 years, except for more complex organic compounds in materials resins, rubber, etc. Special consideration must be given to the effect of high pH on the rate of biodegradation. Studies at Nirex in the United Kingdom (Colasanti, et al. 1991) concluded that natural soil organisms can live at pH levels up to 12.25. and that despite the high pH Aviable populations can exist within regions adjacent to the concrete, e.g. where a surface film coats the concrete. Carbon dioxide and methane will be produced by microbial action within the repository but actual rates of production will be lower than that in a domestic landfill.
The model is based on the assumption of chemical equilibrium within each compartment which is justified because of the long travel times through each compartment. For example travel time through the concrete is estimated at about 40 years if the water flows through fine cracks, and longer if the concrete maintains its integrity.

Fig. 1. Conceptual model for an above grade concrete LLW disposal facility.
Implementation of the Conceptual Model in the Geochemical Code PHREEQC
The geochemical code PHREEQC, version 1.3 (Parkhurst, 1995) was used to simulate changes in the quality of the infiltrating water and the leachate leaving the facility. PHREEQC was used to simulate the dissolution of alkali hydroxides, portlandite, and the C-S-H gel. Portlandite, C-S-H and calcite were specified as the major solid phases in the model. The assumed hydrated cement content of the concrete was 10 percent by weight. According to Berner (1992), typical hydrated cement consists of (by weight): 40-50% C-S-H gel, 20-25% portlandite [Ca(OH)2], 10-20% aluminum and iron oxides, 10-20% pore solution, and 0-5% minor components [NaOH, KOH, Mg(OH)2, etc]. In the present work it is assumed that the C-S-H gel is 40 percent and the portlandite is 20 percent of the cement by weight.
Following Berner (1992), the congruent dissolution behavior of the C-S-H gel is described by describing the C-S-H gel as a non-ideal mixture of two model solids, SiO2 and CaH2SiO4, each of which is treated as a single solid phase. The model assumes that the system reaches equilibrium with portlandite and CaH2SiO4. The thermodynamic constants of portlandite are available in one of the databases in PHREEQC and the thermodynamic constants for CaH2SiO4 are available in Berner (1992). The thermodynamic constants for NaOH and KOH were obtained from the CRC Handbook of Chemistry and Physics (54th Ed.) (Weast, 1973). The values of key input parameters used in the model are given in Table I.
Table I. Input Parameter Values Used in the Geochemical Model
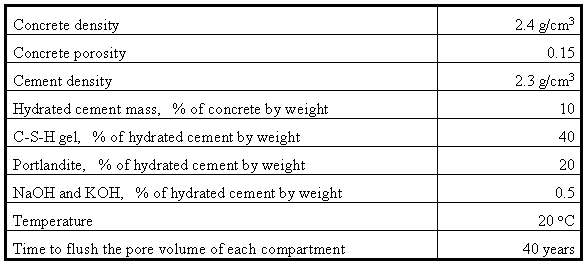
The 40-year time period needed to flush the pore volume of each compartment is from calculations assuming that the concrete deteriorates with time leading to the development of cracks which act as preferential flow paths. The assumed rate of infiltration through the soil cover cap is about 0.5 cm/year. The 40-year period is substantiallyshorter than the time of flow through the same mass of concrete if the concrete maintains its integrity.
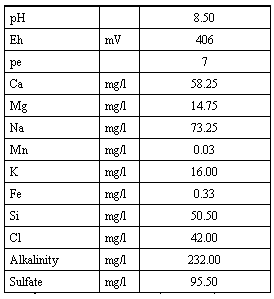
The chemical properties of the infiltrating water were based on shallow ground water samples from the site near Butte, Nebraska and are given in Table II.
Table II. Chemical Properties of Infiltrating Water
SIMULATED pH AND TOTAL DISSOLVED CARBON
The initial pH of pore water in concrete is maintained at about 13.6 due to the influence of alkali hydroxides. Within the modeled volume of concrete, the concentration of NaOH and KOH would be about 0.28 mol/L and 0.21 mol/L respectively. These estimates are based on the assumption that there is 0.5% of NaOH and KOH by weight in the hydrated cement. The PHREEQC modeling results indicate that the pH of the initial pore fluid would be controlled by the complete dissolution of NaOH and KOH. During the first cycle of the pore volume flushing with infiltrating water the pH of the pore water in the concrete is maintained at 13.6. The high pH is maintained for the first cycle of flushing and presumably into some part of the second cycle; therefore, this very high pH would last for about 100 years. During the first cycle, calcite has a positive saturated index, suggesting favorable conditions for its precipitation.
The PHREEQC modeling results show that after the complete dissolution of the alkali hydroxides, the cement pore water chemistry is strongly controlled by portlandite and the C-S-H gel. In the presence of portlandite and C-S-H, the pH of the pore water is maintained at about 12.6. The portlandite dissolves at a rate of 0.024 moles per cycle of flushing (or 0.0006 moles per year). At this rate, it would take about 11,000 years to exhaust all portlandite present in the modeled volume (6.4 mol within a concrete/cement volume containing 1 kg of water). This estimate is based on the assumption that the cement mass is about 10% of the concrete mass. The time frame of 11,000 years represents a minimum number because of its conservative assumption of the amount of cement in concrete.
Under the pH controlled by portlandite, the total dissolved carbon is maintained at 6.52 x 10-6 mol/L (7.8 x 10-8 g/cc). Of the various dissolved carbonates, the two highest species are CaCO3 and CO32-, at a concentration of 5.34 x 10-6 and 1.14 x 10-6 mol/L, respectively. Additional C-S-H also precipitates at a rate of 8.35 x10-4 moles per cycle of flushing.
After the complete dissolution of portlandite, the C-S-H controls the equilibrium pH level at 10.4. In this stage, calcite still has a positive saturation index, indicating that carbonates remain in solid phases. Calcite continues to precipitate at the rate of 0.003 moles per cycle. Under the pH of 10.4 controlled by C-S-H, the total dissolved carbon is maintained at 1.54 x 10-5 mol/L. By the time that pH drops to 10.4 (11,000 years, i.e. close to two half lives), the carbon-14 would have decayed by at least a factor of about 4. Of the various dissolved carbonates, the two species of the highest levels are CaCO3 and CO3--at concentrations of 5.4 x 10-6 and 1.1 x 10-6 mol/L, respectively. Various forms of SiO2 also show up in solid phases. The various forms of SiO2 come from the dissolution of C-S-H.
Under the same assumptions, the C-S-H dissolves at a rate of 0.0038 moles per cycle of flushing (or 0.000095 moles per year). At this rate, it is estimated that it would take about 74,000 years to exhaust the 7 moles of C-S-H present in the modeled volume, assuming that the cement mass is about 10% of the concrete mass. The time frame of 74,000 years represents a minimum number because of its conservative assumption of the amount of cement in concrete.
After the complete dissolution of C-S-H, the pH decreases to about 7.6. The total dissolved carbon species concentration increases to 0.0034 mol/L, and the dominant carbonate species is HCO3- at a concentration of 0.0031 mol/L; CO3-- occurs at a concentration of 7.150 x 10-6 mol/L. Figure 2 gives the simulated pH evolution over time The concrete retains a pH that is representative of Environment I or Environment II for such a long period of time that these environments are appropriate for determining the parameters used in PA modeling.
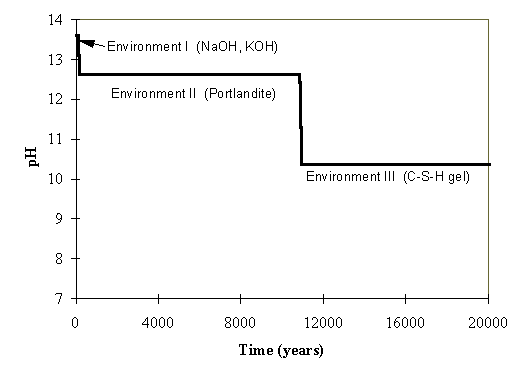
Fig. 2. Simulated pH Evolution as a function of time.
SENSITIVITY ANALYSIS
A sensitivity analysis was conducted to assess the significance of different assumptions used in the model. The sensitivity analysis focused on:
The results of the sensitivity analysis indicate that the pH and the concentration of total carbon in the concrete are not sensitive to any of these parameters. However, the characteristics of the infiltrating water do affect the pe of the leachate leaving the floor slab. If no oxygen is available in the concrete vault, then pe of the water is negative, i.e. reducing conditions prevail. If, however, oxygen enters the pore space then with the same infiltrating water, the pe increases leading to oxidizing conditions.
ESTIMATE OF THE C-14 SOURCE TERM
The dissolved carbon concentrations and calcite concentrations estimated with the geochemical model represent total carbon in the waste/concrete system, which includes all carbon isotopes: C-12, C-13 and C-14. While the amount of C-14 is limited to the initial inventory, the amount of available C-12 is constantly increasing through the generation of carbon dioxide as a result of the biodegradation of organic matter in the waste. The continuous generation of carbon dioxide (12CO2) and the formation and deposition of calcite (Ca12CO3) within the waste and concrete means that the fraction of C-12 to C-14 will continuously increase. Through isotopic exchange between the C-14 in the calcite and the increasing availability of C-12, the fraction of C-14 to C-12 in the solid as well as in the aqueous phases will decrease with time.
The C-14 concentration depends on C-12 availability and simulated deposition rates. Specific results of the geochemical model and other assumptions affecting C-14 concentrations are:
Figure 3 shows the evolution of the C-14 source concentration in the A cells, estimated based on these assumptions.
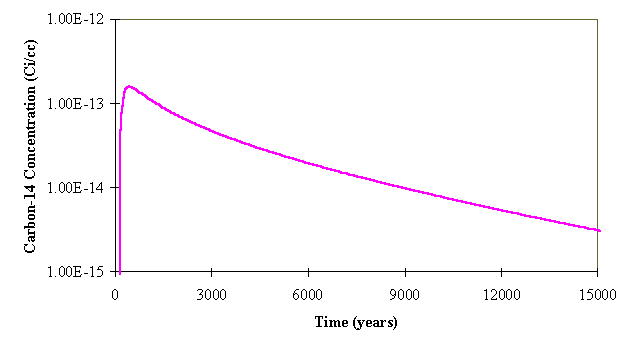
Fig. 3. Estimated C-14 source concentration for the Class A cells.
CONCLUSIONS
A methodology for estimating the C-14 source term in above grade concrete LLW disposal facilities was developed. The model accounts for the effect on C-14 concentrations in the leachate of the constantly increasing available C-12 due to the generation of carbon dioxide as a result of the biodegradation of organic matter in the waste. The continuous generation of carbon dioxide (12CO2) and the formation and deposition of calcite (Ca12CO3) within the waste and concrete means that the ratio of C-12 to C-14 will continuously increase. The paper discusses how through isotopic exchange between the C-14 in the calcite and the increasing availability of C-12, the fraction of C-14 to C-12 in the solid as well as in the aqueous phases will decrease with time.
The application of the model suggests that in the analyzed facility Environment I conditions will exist in the pore water of the bottom slab during a relatively short period (of the order of 100 to 150 years), and Environment II conditions will persist during the rest of the 10,000-year time-frame of the performance assessment analysis. The pH of the pore water in the bottom slab of the CIC facility will remain higher than 12.6 during the 10,000-year time-frame of the performance assessment analysis.
ACKNOWLEDGMENTS
The model described in this paper was developed in support of the performance assessment analysis for the proposed Central Intestate Compact (CIC) low-level radioactive waste disposal facility in Butte, Nebraska (BNI, 1995). Member states of CIC are Nebraska, Kansas, Oklahoma, Arkansas and Louisiana. If approved, the facility will be operated by US Ecology, and will be regulated by the State of Nebraska. Mr. John DeOld is the US Ecology project manager for the CIC facility. Bechtel National, Inc., as a prime subcontractor to US Ecology, is providing siting, licensing, design, safety assessment and construction management services to US Ecology.
REFERENCES