CLEANING OF GROUND CONTAMINATED WITH 226Ra and 238U
L.B.Prozorov, V.V.Martianov, M.Yu.Chtcheglov,
V.B.Nikolayevsky, V.L.Tarasov
Mos SIA "Radon"
ABSTRACT
Main problem of ground decontamination technologies is the most full extraction and multiple reduction of radioactive materials volume which are the subject of RAW placement. The majority of developed methods of grounds cleaning is based on the leaching of radioactive elements (using various chemical reagents) and chemical solutions processing (the result of leaching). Among them an electrokinetic method of ground cleaning including processes of leaching, electrodialisis and electroosmosis takes special place. This method can have certain advantages as compared with other metods, especially in the case of low water permability grounds and soils.
INVESTIGATION
The complex researches on the cleaning of grounds from 226Ra and 238U were conducted in the following sequence:
The researches were conducted in laboratory conditions. Two given samples of the ground ( 20 kgs each) contaminated with radium and uranium were selected. Scale - spectral analysis data of the ground samples are submitted in Tables I and II.
Table I. Radionuclide Structure of the Ground 1.
| Radionuclides | 226 Ra |
238 U |
234 U |
232 Th |
228 Th |
| Specific radioactivity, Bq/kg | 24000 |
6,2 |
7,7 |
45 |
56 |
Table II. Radionuclide Structure of the Ground 2.
| Radionuclides | 226 Ra |
238 U |
235 U |
232 Th |
228Th |
| Specific radioactivity, Bq/kg | - |
6.2 gms/kg |
- |
<1 |
<1 |
The contents of uranium and thorium (ground sample No1) are dozens of times lower than significant amounts. Therefore researches were directed only for radium decontamination. The ground sample No2 is characterized by uranium mono-contamination.
The contents of other (unradioactive) metals was determined in the samples of the grounds (Table III.).
Table III. The Contents of Basic Unradioactive Elements in the Ground (%, weight.)
Ca |
Fe |
Al |
Ti |
K |
Co |
Ni |
Cu |
Zn |
Pb |
0,83 |
2,7 |
4,6 |
0,19 |
1,8 |
0,034 |
0,066 |
0,3 |
0,42 |
0,043 |
STATIC LABORATORY RESEARCHES
The laboratory experiments on determination of various chemical reagents efficiency for ground cleaning from radium and uranium were conducted in static conditions by the way of air - dry ground contact with liquid phase, solutions of salts and acids. Experiment conducted under room temperature conditions in closed glasses using periodic intermixing and mechanical mixer for 24 hours. The ratio of solid and liquid phases (S/L) was 1:5 and 1:2 respectively.
Liquid and solid phases were divided by filtering after the test, and 226Ra and 238U contents in water solution were determined by the scale-spectral method, using the peak multichannel analyzer with Ge-Li detector.
NH4Cl, CaCl2, BaCl2, AlCl3 and FeCl3 solutions, and also hydrochloric and nitric acid were tested as desorption reagents for 226Ra. Nitric acid, potessium and sodium salts were used for 238U desorption. The results of the experiments for 226Ra desorption are indicated in Table IV.
Table IV. Radium Desorption Data (S:L=1:5)
| Chemical reagent | pH of solution (initial) |
pH of solution (end of the test ) |
The contents of radium, Bq/ml |
Extraction, % |
| 1N CaCl2 | 5,7 |
6,3 |
649 |
15 |
| 1N NH4Cl | 6,4 |
7,5 |
800 |
19 |
| 1N BaCl2 | 5,9 |
6,7 |
810 |
19,3 |
| 1N FeCl3 | 1,4 |
1,9 |
3020 |
72 |
| 1N AlCl3 | 3,0 |
3,5 |
2190 |
52 |
| 1N HCl | 0,0 |
0,4 |
3060 |
73 |
| 1N HNO3 | 0,3 |
2940 |
70 |
|
| Mixed reagents | ||||
| 0,5N HCl | 0,4 |
1,2 |
2320 |
55,3 |
| 0,5N HCl + 1N NH4Cl | 0,5 |
1,68 |
2470 |
58,8 |
| 0,5N HCl + 1N CaCl2 | 1,04 |
2580 |
61,4 |
|
| 0,5N HCl + 1N BaCl2 | 1,44 |
1800 |
42,8 |
|
| 0,5N HCl + 1N FeCl3 | 1,15 |
1150 |
27,4 |
The highest degree of 226Ra extraction (70 %) within one stage of processing was reached using 1N solutions of acids and cloride iron.
The large efficiency of three-charge cations of aluminium (52 % extraction) and iron (70 % extraction) and low efficiency of one and two charge cations (NH4+, Ca2+, Ba2+) indirectly specify the ion-changing character of radium sorption by the ground.
The adding of ammonium and calcium salts into hydrochloric solution acid results in insignificant increase of radium extraction with the comparison of mono-acid solution (from 55,3 % up to 58,8 % and 61,4 %). The diminution of the ground cleaning efficiency up to 42,8 and 27,4 % is observed in a case when the barium and iron solutions with the hydrochloric acid are applied.
The obtained outcomes show, that most effective reagents for ground cleaning from radium are the solutions of chloric iron, and hydrochloric acids. Therefore these reagents are chosen for further researches.
The results of uranium leaching (S:L=5:1) showed, that the most effective reagents were as follows:
Application of NaHCO3 has not given a positive effect. The reagents containing SO42--ions, were not applied because the large content of Ca,MgCO3 was in the ground, and CaSO4 changing ground structure was being formed.
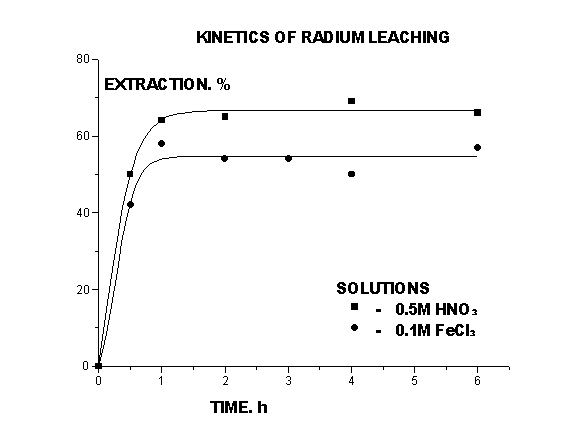
The kinetics of radium leaching (Fig.1.) was investigated. It was found out, that an equilibrium in the ground - solution system is formed enough quickly for 1,5 hours.

Figure 1. Kinetics of Radium Leaching
Researches on uranium desorption have shown, that the balance in the ground - solution system is formed for 1.5-2 hours after beginning the experiment.
The dependence on radium leaching from various concentration of HNO3 and FeCl3 was determined.
The growth of HNO3 concentration up to 0,5 M/l results in sharp growth of radium extraction in the solution (up to 60 % for one stage of leaching, the ratio S:L = 1:5). Then this dependence becomes smooth. The further acid concentration magnification results in maximum radium extraction up to 90 % conteining 2M/l of nitric acid.
The influence of initial concentration of iron salt on radium extraction is observed only up to 0,1M/l (Table V). The maximum 226Ra extraction degree of from the ground comes 74%. The further magnification of concentration does not result in growth of radium transition in the solution.
Table V. Dependence of Radium Extraction from Reagent Concentration (Ground Sample - 15 gms; S:L=1:5)
Concentration of HNO3, M/l |
Concentration of 226Ra in the solution, Bq/l |
General contents of Ra-226 in the solution, Bq |
Radium extraction, % |
Concentration of FeCl3 M/l |
Concentration of 226Ra in the solution, Bq/l |
General contains of 226Ra in the solution, Bq |
Radium extraction, % |
0,1 |
380 |
29 |
8 |
0,01 |
620 |
47 |
13 |
0,2 |
1200 |
90 |
25 |
0,05 |
1920 |
140 |
40 |
0,5 |
2830 |
212 |
59 |
0,07 |
2590 |
194 |
54 |
1,0 |
3450 |
259 |
72 |
0,1 |
3550 |
266 |
74 |
2,0 |
4220 |
317 |
88 |
0,33 |
3450 |
259 |
72 |
3,0 |
4030 |
302 |
84 |
1,0 |
3260 |
245 |
68 |
4,0 |
4270 |
320 |
89 |
- |
- |
- |
- |
The amount of reagents (E = 70 %) comes to about 81g/kg for 0,1M FeCl3 and 315g/kg for 1M HNO3.
It is necessary to note, that other chemical elements also pass in the solution from the ground (TableVI).
Table VI. The Contents of Metals in the Solution, gms/l (After 2M HNO3 Treatment)
Fe |
Ca |
Zn |
Cu |
Pb |
Ti |
Ni |
1,52 |
0,37 |
1,77 |
0,49 |
0,076 |
0,04 |
0,024 |
The experiments on radium leaching at the ratio S:L = 1:2 also were conducted. It was made for reduction of radioactive solutions volumes formed during the ground cleaning.
The fact of diminution of radium extraction degree at the L:S reduction for the majority of reagent concentration was determinated ( except 2M HNO3 solution, where the degree of extraction was ~ 88 %).
It is also necessary to note insignificant decrease of radium extraction from 74 up to 65 % using 0,33M (53,6 g/l) solution of FeCl3. Specific amount of reagents comes to 107g/kg for FeCl3 and 252 g/kg for HNO3.
The repeated processing of the ground was conducted by the fresh reagent solutions for reaching deeper cleaning. 21 % of radium (0,33M FeCl3) and 10 % of radium (2M HNO3) were extracted in addition. The general extraction comes to 86% and 98 % for salt and acid respectively.
Thus, radium leaching at low significances of S:L = 1:2 ensures effective ground cleaning and high radionuclide concentration in liquid phase. The important result is also volume reduction of solutions which are the subject to further treatment.
DYNAMIC LABORATORY RESEARCHES
The data of static researches were used for dynamic column experience (reagent filtration through the ground). The nitric acid (concentration - 31 gms/l) solution was used, as a desorption reagent for 226Ra leaching.
The solutions of nitric (1M/l) and hydrochloric (1M/l) acids and KHCO3 (1M/l) were selected for 238U desorption.
The average velocity of filtration was 0,32 cm/hours.
The experimental data of radium extraction are submitted in Fig 2.
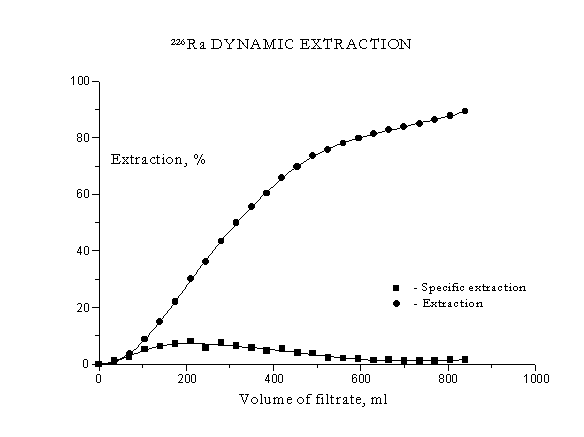
Figure 2. 226Ra Dynamic Extraction
Dependences of 226Ra extraction degree (E %) and its specific radio-activity in the solution (C, Bq/ml) on the amount of filtered reagent (V) were obtained. The dependence of kind C = f (t) has a diffusion maxima (E ~ 8 %) at a volume of filtrate ~ 210 ml, that corresponds to N = 2.4.
The pH significance during the leaching changed from 7 up to 0,3, coming closer to the initial solution pH.
The general radium extraction grows constantly with the N magnification (the cycle number), reaching E=89 %, V = 840ml, i.e. N~10.
Dependences of 238U extraction degree (E %) on the filtered reagent number of cycle (N) were obtained (Fig.3).
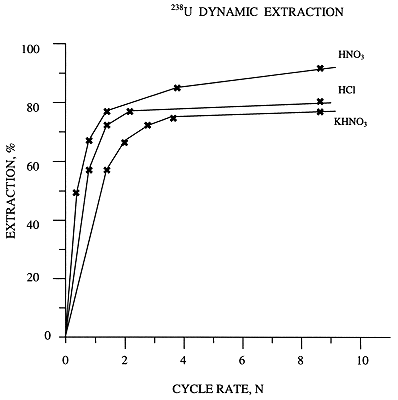
Figure 3. 238U Dynamic Extraction
The peaks of uranium concentration correspond to values N=1.5 (HHO3), N=1.3 (HCl) and N=2.2 (KHCO3). The maximum values of uranium extraction (E %) were achieved at values N=6 (HNO3 -92%, HCl - 82%, KHCO3 - 80%).
ELECTROKINETIC LABORATORY RESEARCHES
The electrokinetic cleaning of the grounds has the stages as follows:
We used process of water electrolysis for shaping ions of hydrogen, which moves from the anode to the cathode and forms an acid medium. It gives a possibility of conducting electrokinetic ground cleaning without adding chemical reagents.
The electrokinetic cell was used for making experiments. The cell consists of three chambers: the working chamber, the cathode chamber and the anode chamber. The special diaphragms separated electrode chambers from the working chamber.
The cation-changing diaphragm (membrane) was used both for prevention of inflow OH-ions into the ground and decrease of the return diffusion stream of cations-radionuclides.
The ultrofiltering diaphragm (membrane) is a neutral filter throagh ions-contaminants from the anode chamber pass freely. Titanium-and-platinum plate was used as anode, and corrosion-proof steel was used as cathode.
The force of the current was constant and was supported automatically: in the first period - 50mA, and after 30 day period - 25mA.
90 % of 226Ra from the ground was extracted for 82 day. 70 % of 226Ra was extracted from the cathode zone and 20 % of 226Ra was extracted from the anode zone. The further electroeffect during consequent 10 day has not reduced in additional 226Ra extraction.
94% of 238U from the ground was extracted for 32 days. It is necessary to note, that significant part of uranium (up to 60 %) migrated as anion form (application of KHCO3). Application of acids resulted in mainly cathion form of uranium migration.
The experiment conducted in laboratory conditions showed a possibility of ground cleaning from 226Ra and 238U up to 90%.
SORPTION LABORATORY EXPERIMENTS
Secondary liquid radioactive wastes form after application of reagent or electrokinetic method of cleaning. The effective 226Ra removal from contaminated solutions can be reached using radium sedimentation with BaSO4. Uranium sorption was not considered.
The check of method efficiency was conducted using two chemical solutions:
These solutions contained way elements as follows: Fe2+3+, Ca2+, Zn2+.
Therefore a smole amount of concentrated sulfuric acid (1 % from volume of the solution) was added in the solutions. Then 2ml of BaCl2 (C = 10g/l.) was gradually added.
More than the 100-multiple SO42- surplus (because of Ba2+ ) was supported during the sedimentation. The time of solution intermixing made 2 hours. After upholding the solution was filtered and than it was analyzed for 226Ra determination. In filtrate 226Ra was not found out within the limits of analysis sensitivity.
The conducted experiments showed a possibility of complicated structure concentrated solutions cleaning from 226Ra using sedimentation method. It has allowed to reduce the volume of solid radioactive wastes dozens of times.
CONCLUSION
The conducted laboratory researches have shown a possibility of ground cleaning from radium and uranium, using both the reagent and electrokinetic methods.
The solutions of nitric and hydrochloric acids, and also solutions containing Fe3+, have the greatest 226Ra extraction efficiency.
The solutions of nitric and hydrochloric acids have the greatest 238U extraction efficiency.
The static (226Ra) researches showed, that the magnification of extraction degree happens to growth nitric acid concentration up to 2M/l (126g/l) and iron sult up to 0,1M/l (16,2gms/l). The maximum significances of 226Ra extraction (88 % and 74 %) are reached during the first stage of leaching. The maximum significances of 238U extraction (68 % and 62 %) are also reached for the first stage of leaching.
The dynamic tests showed, that the initial contamination can be reduced up to 90% (226Ra and 238U). The specific radioactivity of the ground after the experiment has made 1,1.103 Bq/kgs, that is lower than an allowable radioactivity level. The specific amount of HNO3 has made 275 g/kg.
The electrokinetic researches have showed, that the initial contamination of 226Ra and 238U can be reduced up to 90% also. However duration of this process makes dozens of days (especially for 226Ra). Therefore its application is expedient when the collection and transportation of the contaminated ground is impossible (it is IN SITU method). The amount of the electric power have made 3 kW.h/kg.
Sedimentation tests showed, that the 226Ra sedimentation with BaSO4 happens quantitatively. It was experimentally noted, that decontamination of the ground with consequent 226Ra accumulation in the sulphate form allows to reduce the mass of solid radioactive wastes more than 100 times.
REFERENCES