SCALE-UP FOR IN SITU BIOREMEDIATION OF GROUNDWATER CONTAMINATED BY CHLORINATED SOLVENTS
Douglas E. Jerger, Ph.D.
OHM Remediation Services Corp.
Rodney S. Skeen Ph.D., P.E.
Battelle Pacific Northwest Laboratories
Lewis Semprini, Ph.D.
Oregon State University
Daniel P. Leigh, P.G.
OHM Remediation Services Corp.
Steve Grenade, P.E.
U.S. Navy
ABSTRACT
Chlorinated solvents are common groundwater contaminants encountered at many DOE facilities. Most chlorinated groundwater plumes are being treated by pump and treat technologies. This technology is costly, requires long treatment times, and has been termed ineffective by EPA. Pump and treat systems can be combined with and accelerated by in situ bioremediation. This combination can shorten treatment times, reduce costs, and increase destruction of chlorinated solvents. Only minor modifications to the pump and treat system are necessary to allow for nutrient injection to enhance the activity of the naturally-occurring microorganisms. By accelerating their activity, the naturally-occurring microorganisms are capable of degrading chlorinated solvents at concentrations up to 200 ppm and preferentially grow near a DNAPL source.
Scaling laboratory tests to a viable in situ bioremediation field process for the treatment of chlorinated solvents has been challenging since the underlying phenomena are complex. To aid in the scaleup process, OHM and Battelle are developing and applying a rigorous design process that combines laboratory and field characterization activities and reactive flow and transport analysis. This process is being applied to treat PCE and TCE contaminated groundwater at the Naval Air Weapons Station in Pt. Mugu, California. Numerical simulators have been adapted to reflect both site-specific conditions and pertinent microbial metabolisms. The simulators were then used to design and analyze pilot scale field tests to develop estimates for microbial kinetic and hydraulic properties. This information is being compiled to formulate an overall cleanup strategy involving accelerated and intrinsic in situ bioremediation.
INTRODUCTION
In situ bioremediation is one technology being developed to meet the need for cost-effective methods to remediate groundwater contaminated with chlorinated solvents. Effective in situ treatment circumvents the high costs associated with recovering and treating large volumes of contaminated material using pump-and-treat methods. One of the primary technical barriers to widespread application of in situ bioremediation is the inability to accurately account for geochemical, hydrogeological, and microbial phenomena during the design process. Recent bioremediation efforts have focused on this problem by developing and testing simulation tools for scale-up of in situ processes which combine fluid mixing and transport predictions with numerical descriptions for biological transport and reaction kinetics.
Engineering design of in situ bioremediation technologies is conceptually similar to the design of other systems. Specifically, measured or estimated system parameters and constraints are converted into process parameters through engineering design calculations. These process parameters are then used to evaluate the applicability of a technology against performance parameters such as cost and effectiveness. Typical information needed to estimate performance parameters includes the numbers and locations of injection, extraction, and monitoring wells; monitoring requirements; pumping rates; nutrient injection strategies; and the length of time the system must operate.
The success of the design process is determined by the accuracy of the a priori calculations to predict values for the performance parameters. This success requires design engineers to know the critical variables in the design process and use the appropriate level of detail to estimate or measure these variables. In addition, the design engineer needs suitable computational tools to calculate both the process and performance parameters. To aid in this procedure, Battelle Pacific Northwest Laboratory has developed a computer-based engineering design tool BioSolveSM. The design tool is a suite of reactive flow and transport codes, coupled with a design process, that together enable rational scale up and operation of in situ bioremediation systems.
BioSolveSM, an in situ bioremediation design tool developed by Battelle, is used for development and application of in situ chlorinated ethene bioremediation. This design tool is now being validated at multiple sites in conjunction with industrial partners. BioSolveSM allows efficient evaluation of flow, transport, and biological reactions associated with in situ bioremediation processes to effectively optimize the system design and operating procedures. Efficient bioremediation of high concentration areas within groundwater is accomplished by stimulating microorganisms that can use chloroethenes such as TCE directly in energy-producing reactions. Stimulation of this metabolism can result in significantly enhanced destruction rates with better nutrient efficiency when compared to traditional co-metabolic bioremediation processes. Additionally, this technology can be applied for concentrations of chloroethenes greater than 100 mg/L. Complete dechlorination of chloroethenes to the drinking water standard using this metabolism is possible with ethylene and CO2 as the final products. Transport of oxygen to the aquifer is not required because the microbial metabolism occurs under anaerobic conditions.
The objective of this project is to design and demonstrate a cost-effective in situ bioremediation technology to remediate chloroethene contamination in the groundwater at the IRP Site 24 of Naval Air Weapons Station (NAWS) Point Mugu, California.
BACKGROUND
Site History
The Navy has maintained operations at NAWS Point Mugu since 1945. Today, NAWS Point Mugu is the major center for naval weapons systems testing and evaluation. It also provides range, technical, and base support for fleet users and other Department of Defense (DOD) agencies.
Installation Restoration Program (IRP) Site 24 was formerly known as underground storage tank (UST) Sites 23 and 55. These UST sites are adjacent to each other in the western portion of the main base as shown on Figure 1. Because chlorinated solvents were detected at these sites, they were moved into the IRP and are known collectively as IRP Site 24.

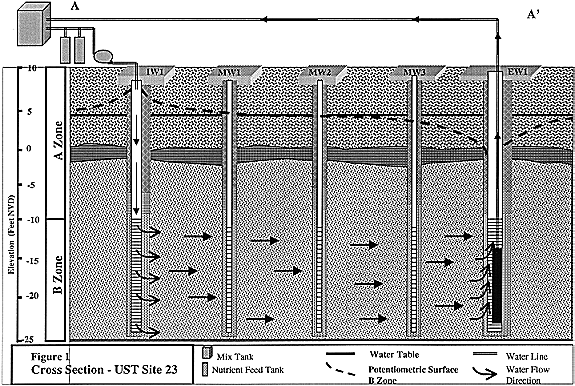
Fig. 1. Cross section - UST Site 23.
At former Site 23, one 550-gallon concrete UST was installed in 1970 and was used as an oil/water separator. Holes were reportedly observed at the joints between two sections of the UST when it was removed in 1989 and a new oil/water separator was installed into the same UST pit. Subsequently two phases of a site inspection (SI) were conducted at the site during which groundwater and subsurface soil was sampled via monitoring wells and Geoprobes. The chlorinated solvents trichloroethene (TCE), tetrachloroethene (PCE), 1,2-dichloroethene (DCE), vinyl chloride, 1,1-dichloroethane (DCA), 1,2-DCA, and 1,1-DCE were detected at the site as well as total recoverable petroleum hydrocarbons (TRPH) and benzene, toluene, ethylbenzene, and xylenes (BTEX).
Former UST Site 55, located to the east of former UST Site 23, contained one 500-gallon steel UST. This UST was used from the late 1950s to the early 1960s to store waste etching solution and washing fluids from the circuit board etching and cleaning activities conducted in Building 352. The chlorinated solvents PCE, TCE, 1,2-DCE, vinyl chloride, 1,1-DCE, 1,1-DCA, 1,1,1-trichloroethene (TCA), 1,1,2-TCA, chloroform, carbon tetrachloride, TRPH, ethylbenzene, and xylene were detected at the site.
Site Characterization
The Mugu Lagoon and its surrounding wetlands are located at the southeastern end of the Oxnard Plain and just west of the Santa Monica Mountains. The Oxnard Plain is underlain by a thick accumulation of late Tertiary and Quaternary sediments. Three general lithologic units are present in the upper 100 feet of IRP Site 24: a coarse to fine grained sand (mostly fill) from surface to approximately 5 feet below ground surface (bgs); a clay layer from approximately 5 to 10 feet bgs; and a coarsening upward sand; silty sand and silt from approximately 10 to 90 feet bgs. A clay unit of undetermined extent or thickness was identified at a depth of approximately 100 feet bgs.
A multiple-aquifer system is present in the Tertiary and Quaternary sediments in the Oxnard Plain. The California Department of Water Resources (DWR 1965) identified the upper three aquifers, in ascending order as, the Mugu, Oxnard and Semi-perched. The Semi-perched Aquifer may have hydrogeolic communication with the underlying Oxnard Aquifer.
The investigation at IRP Site 24 focused on three arbitrary zones in the upper Semi-perched Aquifer identified as the A, B and C Zones (Figure 1). The A Zone was defined as the portion of the upper Semi-perched Aquifer extending from the water table to a depth of approximately 20 feet bgs. The B Zone was defined as the portion of the Semi-perched Aquifer extending from 20 to 40 feet bgs and the C Zone was defined as the portion of the Semi-perched aquifer extending from 40 to 70 feet bgs. The A Zone includes the shallow fill material, the laterally continuous clay and the upper portion of the thick sand and silt unit. The aquifer is completely gradational from the C through to the lower A Zone.
Groundwater is encountered about 5 feet bgs. The potentiometric surfaces of the A, B and C Zones are relatively flat. The site-wide groundwater gradient is approximately 0.001 ft/ft to the south in the A Zone and 0.01 ft/ft to the south in the C Zone. Groundwater generally flows toward a tidally influence drainage ditch immediately south of the site. Locally groundwater flow in the A Zone is affected by man made features including utility lines. Mounding of the water table is occurring in the south-central portion of former UST Site 23, most likely due to leaking water lines. The water table rises approximately 1.5 to 2 feet in the winter months, thereby lessening the impact of the mound. Tidal fluctuations in the drainage ditch affect groundwater elevations in wells close to the ditch but do not measurably affect groundwater movement in the area of the former USTs
The probable source of the contaminants at IRP Site 24 is two tanks installed in the upper A Zone at Site 23 and 55. The pathway for transport of the contaminants from the A Zone to the B Zone has not bee determined. Pump tests at the site indicate the laterally continuous clay encountered from 5 to 10 feet below the present land surface acts as an effective confining unit. However, the integrity of the clay may have been compromised during installation of USTs permitting vertical migration of contaminants from the upper A Zone into the lower A Zone and B Zone.
The contaminants at UST Site 23 and UST Site 55 have been detected in the A and B Zones of the upper Semi-perched Aquifer. However, because of slight upward vertical gradients, increasing water density with depth, and the absence of contaminants detected at depth, vertical migration of contaminants to the Oxnard Aquifer is considered unlikely (DWR, 1965).
Chlorinated solvents (1,1,1-trichloroethane, 1,1-dichloroethane, 1,1-dichloroethene, 1,2-dichloroethane, 1,2-dichloroethene, methylene chloride, tetrachloroethene, trichloroethene, and vinyl chloride) toluene, fuel hydrocarbons, and carbon disulfide were detected at IRP Site 24. Of the chlorinated solvents, only PCE, TCE, 1,1-DCA, 1,2-DCA, 1,1-DCE, 1,2-DCE, and vinyl chloride were detected above their maximum contaminant levels (MCL).
PCE was only detected in four A Zone wells at former UST Site 55 at concentrations ranging from 2.1 m g/L to 45 m g/L. The site hydrogeology suggests that each of these detections may be associated with the leakage of the UST and the associated piping. TCE was detected in five A Zone wells at UST Site 55 in concentrations slightly over detection limits (1.5 m g/L to 6.9 m g/L). TCE was detected in three groundwater wells in the B Zone at former UST Site 23 at concentrations ranging from 3.4 m g/L to 2,700 m g/L (Figure 2).
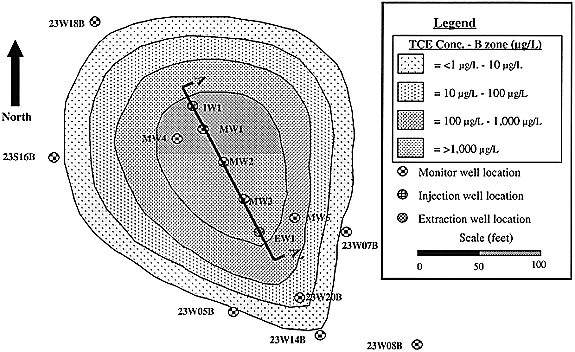
Fig. 2. Plan view of northwestern protion of IRP site 24 showing wells near the TCE plume. Line A - A' corresponds to cross section shown in Fig. 1.
At former UST Site 23, 1,2-DCE was detected at concentrations ranging from near the detection limit to 14 m g/L. The DCE plume has a greater areal extent than the PCE or TCE plumes at both former UST sites. DCE was detected at concentrations near the detection limit to 1,200 m g/L in the B Zone. The highest concentrations of DCE are located to the northwest of the site, collocated with the highest concentrations of TCE. In the B Zone at former UST Site 55, 1,2-DCE was detected in concentrations from 3.1 m g/L to 46 m g/L. The only detection of TCE in the B Zone at UST Site 55 was at the well with the highest 1,2-DCE concentration.
Vinyl chloride was detected in the A Zone at former UST Site 23 from near the detection limit (0.5 m g/L) to 600 m g/L. The highest concentrations of vinyl chloride occur in the vicinity of the former UST. At former UST Site 55, vinyl chloride was detected from near the detection limit to 2.1 m g/L. As with DCE, the extent of the vinyl chloride plume is greater than for the PCE to TCE plumes. In the B Zone, the vinyl chloride plume for the two former UST sites appears to have commingled. Vinyl chloride was detected at a maximum concentration of 7.4 m g/L. Ethene was detected at two locations at IRP Site 24. These locations coincide with the former UST tank location, and the highest concentrations of TCE in the tank vicinity.
APPROACH
In situ remedial alternatives such as bioremediation represent a potentially rapid and low-cost alternative to achieve site cleanup at NAWS Pt. Mugu. Further, an evaluation of bioremediation of chlorinated ethenes at this site could be translated to other similar Navy sites. A thorough evaluation of bioremediation of chlorinated solvents would require a detailed site characterization, and development of site-specific bioremediation rate constants. For these reasons, the Navy chose to assemble a team to evaluate the feasibility of bioremediation of IRP Site 24. This team was chosen because of their ability to measure biodegradation rate constants (Oregon State University), to model bioremediation and design appropriate pilot studies (Battelle), to design remedial actions, communicate with regulatory agencies and coordinate between professional disciplines (Montgomery Watson), and to apply innovative technologies to design/build projects (OHM).
Because the feasibility and ultimate acceptance of bioremediation as a remedial technology depends on site conditions and site-specific information (e.g., the rate of dechlorination of each of the chlorinated ethenes) and bioremediation of chlorinated solvents is an emerging technology, the potential use of this technology for site cleanup was assessed in a step-by-step manner developed by the OHM/RAC Teams.
To initiate the evaluation of the feasibility of bioremediation for site cleanup at this site, the OHM/RAC team conducted a combined laboratory and field investigation of IRP Site 24. OHM and Montgomery Watson conducted the soil and groundwater quality investigation. This field investigation determined the lateral and vertical extent of contaminants and quantified chemical and hydrogeologic parameters affecting the fate and transport of the contaminants. Soil and groundwater samples collected during the field investigation were submitted to Oregon State University where benchscale testing determined site specific bioremediation kinetics. The kinetic values the field data were then applied by Battelle to evaluate bioremediation and natural attenuation using the Biosolve model and to design the pilot test. The determination of the feasibility and potential use of bioremediation at IRP Site 24 will be made based on a variety of factors including:
BENCHSCALE TESTING
Benchscale studies were conducted using groundwater and aquifer solids collected from site monitoring wells and soil borings to assess the feasibility of stimulating indigenous microorganisms in the subsurface to biologically degrade trichloroethylene (TCE) and its lesser chlorinated daughter products. Three different bioremediation schemes were studied: aerobic cometabolism, anaerobic reductive dechlorination, and sequential anaerobic/aerobic treatment.
Studies were conducted in batch microcosms containing site groundwater and aquifer solids. Aerobic TCE cometabolism studies were conducted comparing three primary substrates (methane, phenol, and propane) which have previously been shown to stimulate oygenase enzyme systems and the subsequent degradation of TCE. Maximum TCE removals were only 50 to 60% for methane and phenol utilizing systems following repeated stimulations. Propane utilizing systems showed no apparent TCE removal after two stimulations.
Anaerobic studies were conducted comparing benzoate, lactate, and methanol as potential growth substrates and precursors to fermentation products which could act as terminal electron donors in the dechlorination process. Anaerobic dechlorination studies with benzoate showed only minimal dechlorination of TCE following 130 days of incubation. Lactate amended microcosms, under the same conditions, initiated dechlorination between 15 and 35 days resulting in the dechlorination of all available TCE to VC within 60 to 70 days. The VC in these microcosms is being dechlorinated to ethylene at a slow, steady rate. Methanol (MeOH) amended microcosms responded with rapid MeOH uptake and subsequent methanogenesis following 15 to 20 days of incubation. Minimal dechlorination was observed for these microcosms with the formation of only small amounts of cis-1,2-dichloroethylene (c-DCE) and 1,1-DCE.
Sequential anaerobic/aerobic studies consisted of an aerobic VC-methane cometabolism test in addition to a benzoate amended anaerobic/aerobic test. Aerobic cometabolism tests showed that VC could be effectively degraded to below detectable limits in the presence of methane utilizers. The sequential anaerobic/aerobic test indicated that oxygen requirements, to oxidize the sulfides produced during the anaerobic benzoate process, would be unrealistic under in situ conditions. No methane utilization or VC removal was noted during oxygen uptake in this bottle over a nine day period.
In summary, the benchscale test results indicate that an accelerated anaerobic in situ treatment process with the addition of lactate as the initial electron donor would be the most effective alternative. Lactate stimulation under anaerobic conditions appears to be the most viable approach because of the presence of a strong lactate utilizing-sulfate reducing culture with the ability to completely dechlorinate TCE to ethylene at the site. The accelerated transformation of TCE to VC and the subsequent dechlorination to ethylene for the nutrient media amended tests indicate that nutrients have a substantial effect on the rate and extent of dechlorination. Therefore, nutrient addition may also prove beneficial for pilot and full scale in situ biological treatment.
BIODEGRADATION MODELING
The objective the biodegradation modeling is to design and demonstrate a cost effective bioremediation technology to remediate chloroethene contamination in the groundwater at the IRP Site 24 of NAWS Point Mugu. The primary focus of this work is on accelerated in situ bioremediation. However, existing field data are also being evaluated to estimate natural attenuation rates.
Battelle developed a numerical representation of the spatial distribution of pertinent hydraulic and chemical properties. Pertinent properties are those that affect bioremediation technologies. This numerical description is the basis for using reactive flow and transport analysis in system design. A detailed description of the computational approach, and accuracy of the design simulators have been published elsewhere (Clement et al. 1995; 1996; 1997).
Battelle used BioSolveSM to design a pilot test and develop an operating protocol for conducting a pilot scale in situ bioremediation test. First a numerical representation of the pertinent hydraulic, chemical, and biological properties was developed from field and laboratory data. Battelle integrated microbial kinetic data into BioSolveSM . The source of the kinetic information was obtained from laboratory treatability tests conducted at Oregon State University (OSU). This computational platform was used to simulate different nutrient feeding strategies. Using a baseline injection strategy estimated from the laboratory tests, initial simulations were completed and bioremediation performance was evaluated. Evaluation criteria included the extent of the biologically active Zone and the steady state contaminant destruction rate. Further simulations were then used to maximize both destruction rate and the size of the biologically active zone through refining the nutrient injection strategy. Once an optimum nutrient injection strategy has been identified, locations for additional monitoring wells were chosen based on the spatial distribution of biological activity.
The final step in the design phase was to simulate the full remediation system using the optimized feeding and recirculation strategy. This evaluation focused on the amount of nutrient breakthrough occurring at extraction wells, the level of hydraulic containment, the extent of the biologically active zone, and the overall contaminant destruction rate. Based on this evaluation, the operating and monitoring strategy was refined. Results from this stage in the design process include: 1) the final layout and spacings in the well network; 2) recirculation, nutrient feeding, and monitoring strategies for each phase of operation; and 3) the approximate duration of each operating phase.
IN SITU BIOREMEDIATION DESIGN
Objectives
The purpose of this pilot-scale in situ bioremediation system at IRP 24 is to provide a method to collect field data to validate complete biological destruction of chloroethene groundwater contamination. In addition, the data must provide a sound technical basis for comparing in situ bioremediation with other groundwater treatment processes. To follow, three test objectives were established to meet this goal.
The B Zone at UST Site 23 was selected as the location of the enhanced anaerobic bioremediation pilot test. This location was selected because conditions are most favorable to evaluate complete degradation from TCE to ethene and to collect sufficient data to allow scale-up design for a complete remedial system. PCE and TCE were detected only at low concentrations (< 50 m g/L and <10 m g/L respectively) in both the A and B Zones at UST Site 55. Groundwater modeling indicates that natural attenuation will reduce the chloroethene plume at UST Site 55 to below remedial goals. The highest concentration of TCE was observed in the B Zone at UST Site 23 (2,700 m g/L). Lower concentrations of 1,2-DCE and vinyl chloride (1,200 m g/L and 1.8 m g/L respectively) were observed in the B Zone indicating ongoing biodegradation of TCE by indigenous microflora. In contrast, however, TCE was not detected in the A Zone at UST Site 23 while elevated concentrations of 1,2-DCE and vinyl chloride (1,500 m g/L and 600 m g/L ) were detected indicating considerable intrinsic biodegradation of TCE. Therefore, acceleration of the biodegradation rate would be necessary to achieve the treatment goals.
Another consideration in the selection of the B Zone at UST Site 23 was the ability to evaluate the effect of the pilot test in the aquifer. Aquifer characteristics, including grain size distribution and hydraulic conductivity, are more uniform in the B Zone. The uniformity of these characteristics permits a more accurate determination of fate and transport of injected substrate and anticipated changes in aquifer characteristics. The accurate determination of these parameters will be required to expand the design to include the entire chloroethene plume.
System Design
The design of the well network for the pilot scale test was chosen to provide maximum use of existing wells within the highest chloroethene contaminated region of IRP Site 24. As shown in Figure 2, which is a plan view of the former UST Site 23, the design is based on using 23MW15B as the injection well (I1), 23MW01B as the extraction well (E1), and EW01B as a monitoring location (MW3). The four new monitoring wells shown are necessary since the closest existing wells are outside the zone of influence of the test.
Three monitoring locations between the injection and extraction wells are included in the design since this is the region where much of the biological activity will occur. The two off-center wells will aid in monitoring changes in flow paths caused by biological activity. This information is necessary to assess whether irreversible aquifer plugging occurs.
All wells will have a 15-foot screened interval that begins below the clay layer and extends into the B zone of the aquifer. During operation, groundwater will be extracted at 10 gpm from 23MW01B, amended with electron donor, and reinjected into 23MW15B. The gpm flow rate was chosen to provide high hydraulic control and give an appropriate residence time in the flow field for biological reaction. The effects of recirculation rate on hydraulic control (equivalent to C/Cinject) were evaluated by simulating the changes in concentration of a non-reactive tracer at the extraction well following continuous addition at the injection well. The simulation showed that, at above 5 gpm the level of hydraulic control approaches 85% while a substantially lower level is obtained at a recirculation rate of 1 gpm.
Process Equipment
The primary components of the aboveground system are as follows: substrate injection equipment, automated sampling equipment, process control equipment, and the data management system.
Sampling equipment was designed to obtain representative samples for groundwater for analysis of chloroethenes, anions, and microbes. Dedicated in-well pumps will be used to pump water to the surface for obtaining samples from each monitoring well. Groundwater will also be sampled from ports in the surface piping that connects the extraction and injection wells (recirculation piping). Manual samples for chloroethenes, anions, and microbes will be collected from each sampling location using a syringe and specially-designed sampling ports on the sample lines.
Operation of the in situ bioremediation system requires process control for nutrient injection and sample collection, as well as data management for process monitoring equipment. There are two main equipment systems where process control is important: the lactate injection system and the auto sampler.
The computer control system will be used to turn on, or off, the feed pumps, and adjust their flow rate as appropriate. The feed pumps, the feed-line pressure, the feed-line flow rate, and the feed tank liquid level will all be automatically controlled or monitored. The feed line pressure and injection flow rate will be monitored to determine: 1) that the proper amount of nutrient is being delivered to the well, 2) that there is no plugging of the feed lines, and 3) that there are no leaks in the feed lines. If an adverse condition is encountered an alarm will be activated on the computer and the feed pump will be shut down (if appropriate). The feed tank liquid level will be monitored for a low liquid level, which may indicate siphoning or a leak in the feed tank. A low liquid level condition will also activate an alarm on the computer.
System Operation
The pilot-scale test will be conducted in three phases: an abiotic recirculation control phase (Phase 1) and two biologically active phases (Phases 2 and 3, respectively).
Phase 1, Recirculation Control Phase
The purpose of this phase is to provide data on the variation in aqueous concentrations of chemical species prior to biostimulation. In addition, system trouble-shooting will take place during this period to ensure proper operation during the more critical nutrient injection phases.
Groundwater chemistry data will be monitored at the indicated wells for the duration of Phase 1. In addition, a bromide tracer test will be conducted after 2 weeks of recirculation to provide data to calibrate the flow-and-transport model to aid in process control and data evaluation in subsequent phases. The assumed detection limit of bromide (5 mg/L by ion chromatograph [IC]) for the case where Cinject is equal to 2000 mg/L. Comparison of the detection limit to the predicted responses suggest that a measurable level of bromide should be observed in all monitoring wells.
Phase 2, Startup Phase
The objective of the startup phase is to lower the sulfate concentration within the flow field to allow fermentation and methanogenic process to dominate during Phase 3. This will be achieved by continuously recirculating the flow field at 10 gpm while adding periodic pulses of lactate. This feeding process will continue until the sulfate level at monitoring well MW3 is below 100 mg/L. Simulations predict that this sulfate level will be achieved after 50 days.
Phase 3, TCE Remediation Phase
This phase of the pilot-scale test will rely on a fed-batch operating strategy to achieve measurable contaminant destruction in the flow field without fouling the injection and or extraction wells. Each addition of lactate will be comprised of one high concentration pulse. Pumping will be stopped after 24 hours of recirculation to allow the injected electron donor to fully react to methane. The time between lactate injections will be determined by sampling the groundwater at each monitoring location until acetate is no longer detected (Figures 3 and 4).
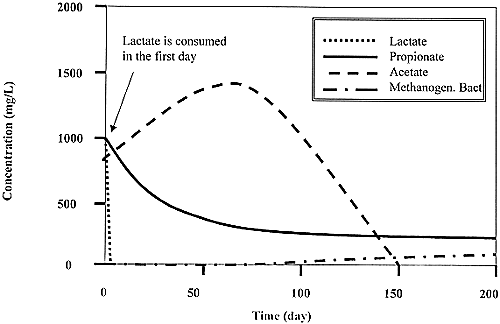
Fig. 3. Predicted temporal changes in the concentrations of lactate, propionate, acetate and methanogenic bacteria during the TCE remediation phase of the pilot-scale test.
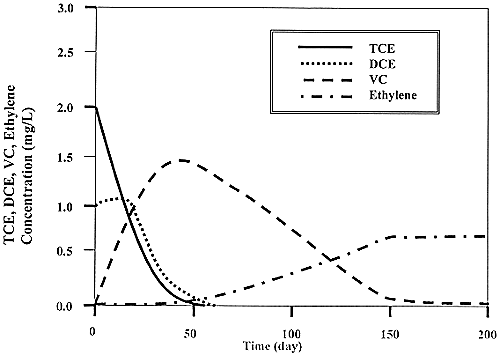
Fig. 4. Predicted temporal changes in the concentrations of chloroethenes during the TCE remediation phase of the pilot-scale test.
CONCLUSION
Efficient bioremediation of high concentration areas within groundwater is accomplished by stimulating microorganisms that can use chloroethenes such as TCE directly in energy-producing reactions. Stimulation of this metabolism can result in significantly enhanced destruction rates with better nutrient efficiency when compared to traditional co-metabolic bioremediation processes. Additionally, concentrations of chloroethenes greater than 100 mg/L can be treated. Complete dechlorination of chloroethenes using this metabolism to the drinking water standard is possible with ethylene and CO2 as the primary products. Transport of oxygen to the aquifer is not required because the microbial metabolism occurs under anaerobic conditions.
The OHM/RAC team can effectively apply in situ bioremediation through use of BioSolveSM, an in situ bioremediation design tool developed by Battelle. This design tool is now being validated at multiple sites in conjunction with industrial partners. BioSolveSM allows efficient evaluation of flow, transport, and biological reactions associated with in situ bioremediation processes to effectively optimize the system design and operating procedures. Coupling the design tool approach with use of a newly discovered, efficient microbial metabolism will streamline the field application of this technology.
Application of chlororespiration to in situ bioremediation is a unique technology to the OHM/RAC team. Preliminary evaluations of the process suggest that cost savings of up to 70% are possible when compared to the baseline pump-and-treat technology.
Direct beneficiaries of this project will include the project team, and the Navy. The OHM/RAC team will benefit by having access to cost and performance field data that can validate the technology. Field data is crucial for commercializing the process since it provides a true evaluation of system performance. The Navy will benefit from the project by having access to a faster and less expensive method for remediating chloroethene contaminated groundwater.
REFERENCES