CEMENTATION OF RESIDUE ION EXCHANGE RESINS
AT ROCKY FLATS
Donald F. Dustin, Thomas D. Beckman, and Catherine M. Madore
Safe Sites of Colorado, L.L.C.
ABSTRACT
Ion exchange resins have been used to purify nitric acid solutions of plutonium at Rocky Flats since the 1950s. Spent ion exchange resins were retained for eventual recovery of residual plutonium, typically by incineration followed by the aqueous extraction of plutonium from the resultant ash. The elimination of incineration as a recovery process in the late 1980s and the absence of a suitable alternative process for plutonium recovery from resins led to a situation where spent ion exchange resins were simply placed into temporary storage.
By the mid-1990s, temporary storage had extended for a minimum of eight years. The storage containers were typically large diameter cardboard tubes, wrapped in plastic, and placed in 55-gallon drums. Depending on the extent of washing that took place just prior to taking the spent resin out of service, varying amounts of residual nitric acid remained absorbed in the resin matrix. The intimate mixture of nitric acid, a strong oxidizer, and the resin matrix, a fuel, represented a potential fire hazard and led the Defense Nuclear Facilities Safety Board to consider ion exchange resins as one of the highest hazard residue materials stored at the Site.
In 1996, Rocky Flats began processing potentially unstable ion exchange resins by combining small amounts of resin into a drum of cement used to stabilize aqueous waste liquids. The resulting product effectively eliminated any ignitability hazard associated with the resin and met all interim storage and waste disposal criteria. An additional advantage was that, by cementing the resin in conjunction with the cementation of liquid wastes, the resin was effectively cemented at a wattage limit that was ten times higher than the wattage limit assigned to a cemented product where ion exchange resins alone were immobilized.
The effort to prepare stored resin for stabilization via cementation with aqueous waste liquids began in April 1996. To date, 100% of the inventory of residue ion exchange resins has been neutralized and repackaged in preparation for the cementation operation, and approximately 35% of the resin has been cemented. Work continues to assay recently repackaged resin and to cement the repackaged product. The cementation effort is expected to continue for about one more year at which time all residue ion exchange resins will have been eliminated as a discrete residue form from the Site.
INTRODUCTION
The Rocky Flats Environmental Technology Site near Denver, Colorado is currently storing a backlog of approximately 100 metric tons of plutonium- bearing residues. Residues comprise a category of materials with sufficiently high concentrations of plutonium that the recovery of that plutonium was, at one time, considered to be economically more favorable than the production of new plutonium in a reactor facility. With the change in mission for Rocky Flats from weapons production to environmental restoration and waste management, plutonium recovery operations will not be required for the foreseeable future. The problem remains, however, as to the ultimate disposition of the residue backlog.
Residues consist of a variety of materials that are by-products of nearly forty years of weapons production. Typical residues are incinerator ash, pyrochemical salts, combustibles (paper, cloth, and plastic), metal, glass, sludges, casting materials, insulation, firebrick, filters, and ion exchange resins. The average plutonium concentration in these materials is about 3 wt%. At Rocky Flats, residues and wastes are given a unique identifier known as an Item Description Code (IDC). There are currently 99 residues IDCs awaiting disposition. Approximately 45% of these residues have been determined to be hazardous as defined by the Resource Conservation and Recovery Act (RCRA) and, as such, are subject to regulation as mixed waste.
This report describes the method that Rocky Flats is currently using to stabilize residue ion exchange resins. The objective of the resin stabilization program is: 1) to ensure their safety during interim storage at the site, and 2) to prepare them for ultimate shipment to the Waste Isolation Pilot Plant (WIPP) in New Mexico. Included in the discussion is a description of the safety concerns associated with ion exchange resins, alternatives considered for their stabilization, the selection of the preferred treatment method, the means of implementing the preferred option, and the progress to date.
BACKGROUND
Ion exchange resins are fine beads of polymeric (plastic) material consisting of styrene and divinyl benzene monomer units. Although bead size varied with use, the typical particle size was in the range of 20 to 100 mesh. Plutonium purification was accomplished by the selective adsorption of plutonium ions (either Pu+3 ions on cation exchange resins or Pu(NO3)6-2 ions on anion exchange resins). Resins were loaded into an ion exchange column where the adsorption process took place. The adsorbed plutonium was eventually eluted from the column using 0.35M nitric acid for anion exchange resins or, in the case of cation exchange resins, 7.0M nitric acid.
Resins in ion exchange columns were periodically replaced and the spent resin stored for eventual plutonium recovery. During the replacement operation, some of the resins were either washed with water (producing leached ion exchange resins, IDC 431) or packaged directly (producing unleached ion exchange resins, IDC 430). In 1996, Rocky Flats was storing approximately 267 kilograms of residue ion exchange resins containing over a kilogram of plutonium.
Resins were drained of all free liquid before being packaged into residue drums, so they are not considered to have the RCRA characteristic of corrosivity. Furthermore, samples of resins have been subject to Toxic Characteristic Leaching Procedure analyses, and none of the samples yielded results above regulatory thresholds for toxic metals. Therefore, ion exchange resins are not regulated as RCRA hazardous wastes.
Although the spent resins are not considered a hazardous waste, there is concern that the intimate contact of an oxidant (nitric acid) and an organic substrate (the polymeric beads) may, over time, result in the generation of products which could have a reduced ignition temperature thus presenting a potential fire hazard. In 1993, the Defense Nuclear Facilities Safety Board (DNFSB) began investigating the safety of residues being stored at Rocky Flats. Their conclusions, published in DNFSB Recommendation 94-1 in May 1994, stated that possibly unstable residues should be stabilized to eliminate potential flammability, pyrophoricity, and reactivity concerns and packaged to ensure safe storage until such time as they could be shipped off site (Reference 1). Residues believed to present high risks to operating personnel were to be stabilized within three years while the stabilization and/or repackaging of lower hazard materials was to be completed in seven years.
Ion exchange resins were considered by the DNFSB to be enough of a flammability hazard that their continued storage at the site represented a significant risk to plant workers. Thus, they were designated as one of a few types of residues that should be considered for expedited treatment, well in advance of any other treatment operations that would require extensive upgrades to capital facilities to implement. In 1994, Rocky Flats embarked on a program to investigate candidate processes to eliminate any potential for an adverse oxidizer/fuel reaction leading to the generation of readily ignitable materials.
The safety concern with ion exchange resins is based on the knowledge that spent resins contain significant quantities of nitrates which may react exothermically with the organic matrix of the resin. Nitrates within the resin matrix could be present in one or more of three chemical forms. First, nitro groups (-NO2) could be covalently bonded to the organic matrix forming nitro-organic compounds. This situation is not likely since the conditions necessary to effect the nitration reaction never existed. Such a reaction requires the presence of a mixture of concentrated nitric and sulfuric acids.
The second possible form of nitrates within the resin matrix is inorganic nitrate ions (NO3-1) bound to exchange sites. In anion exchange resins, positively charged exchange sites are ionically bound to anions such as nitrate ions. The exchange process substitutes negatively charged plutonium complex anions for nitrate ions. The regeneration of resin columns reverses the process leaving nitrate ions bound to the resin. Washing the resin with water after use will leave some if not most of the nitrate ions in place; therefore, there is a high probability that ionically bound nitrate ions are present in the resin.
The third form of nitrate within the resin matrix is as interstitial nitric acid (HNO3). Resins are very porous by design, since effective ion exchange requires high surface area and intimate contact between dissolved species and the resin exchange sites. Unleached ion exchange resins are highly likely to contain interstitial dilute nitric acid even though the resin itself may not contain obvious free liquid. Leached ion exchange resins, which have been washed with water prior to removal from the column, will have a lower, but not zero, probability of containing interstitial nitric acid.
TREATMENT AND DISPOSAL OPTIONS
The development of potential treatment options to stabilize ion exchange resins was focused on two sets of requirements. The first set of requirements was embodied in the WIPP Waste Acceptance Criteria (WIPP/WAC) which defined the material form and packaging requirements for all transuranic (TRU) waste to be disposed of in WIPP (Reference 2). Since WIPP is envisioned as the ultimate repository for all Rocky Flats residues, any treatment process undertaken should produce WIPP-certifiable wastes without the need for additional processing at some later date.
The second set of requirements was published as the Interim Safe Storage Criteria (ISSC) which was developed by DOE in response to the DNFSB Recommendation 94-1 (Reference 3). The ISSC defined material form and packaging requirements for plutonium-bearing materials that would be stored at the various DOE sites until such time as WIPP opened.
An initial draft of the ISSC directed that hydrogenous materials such as ion exchange resins did not constitute an acceptable waste form for safe interim storage at DOE sites. Consequently, the process options considered for stabilizing such residues included various matrix destruction techniques. Candidate processes such as incineration, pyrolysis, a variety of novel thermal oxidation techniques, catalyzed electrochemical oxidation, catalyzed chemical oxidation, and acid digestion were identified and evaluated. The final version of the ISSC, however, allowed that hydrogenous matrices could be stored safely if all other hazards were eliminated and provisions were made to prevent the accumulation of flammable gases from radiolysis.
The elimination of matrix destruction as a requirement for the stabilization of hydrogenous materials meant that simpler, cheaper, safer, and more expeditious processing techniques could be employed to meet DNFSB expectations. For ion exchange resins, three candidate processes were identified by the Residue Stabilization organization at Rocky Flats: resin denitration, direct cementation, and cementation in conjunction with an existing liquid stabilization operation. These three processes are described in the following paragraphs.
Resin Denitration
The resin denitration process takes advantage of one of the chemical properties of resins, i.e., the ability to undergo an exchange of one ion for another depending on concentration and the relative affinity of the two ions to be bound to the ion exchange sites within the resin matrix. Experimental work supporting the development of this process was performed by the Los Alamos National Laboratory (Reference 4).
To begin the denitration process, the resin is first slurried in an aqueous solution of 2M sodium salicylate [Na+C6H4(OH)(COO)-]and 1M sodium hydroxide (NaOH). The resulting slurry is transferred to an ion exchange column 5 inches in diameter and 2 feet in length. The column is eluted (washed) with approximately two bed volumes of additional sodium salicylate followed by two bed volumes of deionized water. The washed resin is then removed from the column, dried to eliminate all traces of free liquids, and repackaged for storage or disposal.
The liquids generated from the process are collected, and the pH is adjusted to a value less than 2 by the addition of 6M nitric acid. The neutralization of the alkaline wash solution will result in the precipitation of salicylic acid which is then removed from the resulting slurry by filtration. The salicylic acid is then redissolved in 2M sodium hydroxide to be used for denitration of the next batch of resin. The filtrate from the salicylic acid precipitation (which contains nitric acid, other impurities originating in the resin, and approximately 0.01M salicylic acid) is disposed of as plutonium-contaminated liquid waste.
Direct Cementation
Direct cementation is accomplished by mixing resin, dry portland cement, and water in the appropriate proportions to generate a grout which will subsequently cure into an acceptable monolith. Experimental work supporting the development of the process control parameters for the cementation operation was also performed by the Los Alamos National Laboratory (Reference 5).
Resin is first removed from its current packaging, and approximately 750 grams are placed in a mixing bowl for a standard industrial-sized electric mixer. Water is added to the resin, and the pH of the resulting slurry is determined with a pH meter. Sodium hydroxide is added to the slurry to adjust the pH to a value between 9.0 and 10.5. Additional water (up to a total of approximately 2 liters) and approximately 5 kilograms of dry portland cement are added to the mixing bowl. The resulting mixture is stirred until a homogeneous grout containing about 10 wt% resin is obtained. The grout is then poured into a metal can for curing, packaging, storage, and disposal. The only liquid waste generated from this operation is that due to spills and clean-up activities.
Cementation in Conjunction with Liquid Stabilization
A modification to the direct cementation process is achieved by incorporating the resin material into an existing cementation operation being used to stabilize plutonium-contaminated liquids. This modification is made possible by a provision of the TRAMPAC Waste Codes which allows for the addition of up to 1 wt% of ion exchange resins as an impurity to drums of cemented aqueous waste (Reference 6). The process described here is based on an approved procedure for the cementation of aqueous waste in the Building 774 Bottle Box.
Bottle Box operations are initiated by the preparation of a drum of a mixture of dry portland cement and dry Ramcote 1200 cement. The drum is then affixed to a port in the floor of a glove box in Building 774. In parallel, approximately 60 liters of aqueous waste (containing a maximum of 70 grams of plutonium) are batched for treatment. The batched liquids are transported to Building 774 in 4-liter plastic bottles where they are bagged into the glove box, transferred via vacuum to a feed tank, neutralized (if acidic), and then added to the drum of dry cement. Manual stirring of the liquid-cement mixture produces a grout which is then left to cure producing a cemented waste form containing no free liquids and no respirable fines.
The existing Bottle Box operation would be modified by including, in the batching process, a bottle of neutralized ion exchange resin weighing up to 2 kilograms and containing a nominal 10 grams of plutonium. The plutonium content of the batched liquids would then have to be decremented by 10 grams to ensure that the total plutonium in the final waste product does not exceed 70 grams. Both the liquid waste and the resin would be mixed into the cement at the same time. The cured drum of cement would then be stored and eventually disposed of as TRU waste.
EVALUATION OF ALTERNATIVE PROCESSES
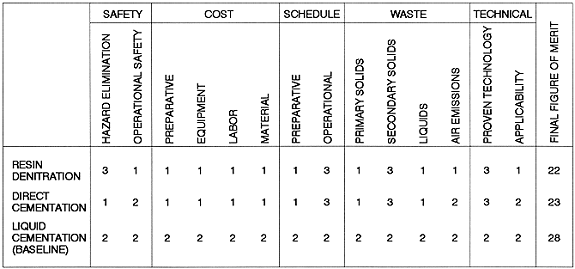
The three alternative processes described above were assessed using a qualitative, relative comparison of the merits of each process. To complete this analysis, fourteen evaluation criteria were identified. These criteria were, in turn, grouped into five categories: safety, cost, schedule, waste generation, and state of technical development.
The assessment of the relative safety considerations included, first, the effectiveness of a particular process in eliminating the known hazards associated with nitrated ion exchange resins. Also considered was the relative safety of the stabilization operation with respect to the degree of risk to be assumed by the operating staff. Considerations included radiation exposure and reagent handling.
The relative costs of the three candidate processes included preparation costs, equipment costs, labor costs, and material costs. Preparation costs included those necessary to write procedures, train operators, and obtain the necessary authorization basis to conduct operations. Equipment costs included design, procurement, and installation costs. Labor costs related to the operating and support staff necessary to perform the stabilization operations. Material costs included reagents, supplies, and other expendable materials.
Schedule considerations included preparation time and operating time. Preparation time was concerned with the time necessary to put all systems in place to initiate stabilization operations. Considered in this criterion are the times required for the design and installation of equipment, the development of procedures, the training of operators, the completion of the appropriate level of NEPA documentation, and the establishment of an authorization basis for operations. Operating time was determined to be inversely proportional to the expected throughput of a particular process.
Waste generation was concerned primarily with the volume of waste into which the resin would be transformed by the stabilization operation. The limiting factor in determining final waste volume was the wattage limit associated with a drum of stabilized resin. Secondary waste volume; consisting of discarded packaging materials, expended equipment, and supplies; comprised the secondary solid waste stream. Liquid waste generation was a criterion as well as the relative amounts of gaseous emissions of environmental concern.
The degree to which the particular stabilization technique had been developed and demonstrated was a criterion. Also considered was the applicability of the process to residue materials other than ion exchange resins.
EVALUATION RESULTS
The cementation of ion exchange resins in conjunction with the cementation of aqueous wastes was determined to be the stabilization process of choice. The criteria that were instrumental in arriving at this conclusion were costs (all types), the preparation time required to initiate operations, and the volume of both liquid and solid waste generated. Figure 1 is the decision matrix that was used to assess the evaluation criteria and arrive at the recommended course of action. For purposes of this evaluation, resin cementation in conjunction with liquid cementation was assumed to be the baseline and was given a rating of 2 for each criterion. The resin denitration and direct cementation processes were given a rating of either 1, 2, or 3 depending on whether they were evaluated as being worse than, the same as, or better than the baseline, respectively. All criteria were weighted equally. The results of this assessment showed that liquid cementation was the preferred course of action.

Figure 1. Resin Stabilization Comparison Matrix
The preparations required for the adaptation of the Bottle Box operation to incorporate ion exchange resins included the revision of existing procedures, incremental training of operators, and the modification of the existing authorization basis. Costs associated with initiating these activities for a new nuclear operation were significantly greater. Liquid cementation equipment had already been purchased, and no new process equipment was needed. Labor costs were to be shared with the Liquid Stabilization program, and only incremental costs to prepare resins for cementation were required. Very little in the way of additional material costs were identified.
The time required for the revision of existing procedures, incremental training of operators, and the modification of the existing authorization basis was estimated to be completed three months sooner than the time estimated for accomplishing the same objectives for a new process. Hence, stabilization of a residue considered to be a high-risk material by the DNFSB could begin that much sooner.
Cementation of resins along with aqueous wastes would generate no additional liquid wastes, a key factor at a plant where liquid waste treatment capability was diminishing. But a primary consideration that led to the selection of this process over direct cementation and denitration was the volume of solid waste to be generated. The products of either resin denitration or direct cementation would have to be packaged in a manner where the decay heat was limited to about 0.02 watts per drum. This limit was established as part of the WIPP/WAC to ensure that the radiolytic generation of hydrogen or other flammable gases would not represent a fire or explosion hazard. Hence, the 267 kilograms of stabilized resin would eventually be repackaged into approximately 200 WIPP-certifiable 55-gallon drums.
The wattage limit for cemented aqueous waste, which allowed the presence of resin as an impurity, was established by WIPP requirements as 0.2 rather than 0.02 watts per drum. Therefore, the cemented resin would effectively be packaged at the higher wattage limit, and only an additional 20 drums of cemented product, above what would have been generated by the Bottle Box anyway, would be generated. Thus, there were distinct advantages in terms of waste generation, storage, certification, and disposal that could be realized by incorporating ion exchange resins into the existing Bottle Box operation.
IMPLEMENTATION OF PREFERRED TREATMENT PROCESS
Once the evaluation of alternatives was completed and the preferred method of treatment identified, implementation of that treatment process was initiated. Several individual operations had to be functional before any cementation could begin. These operations included movement of resin drums to a central repackaging facility, neutralizing and repackaging resins in preparation for cementation, movement of repackaged drums of resin to the Bottle Box, and finally, the cementation operation itself.
In 1996, resins were stored in several buildings at Rocky Flats. Because the current packaging configuration was not amenable to the Bottle Box operation, resin drums were shipped to an existing glove box line in Building 707 where they were repackaged. Initially, 20 out of 22 drums of resins were repackaged in this manner. Two drums remained in their current packaging configuration through FY1997 since they were stored in areas that were infracted due to criticality safety concerns. Those two drums were repackaged in November 1997.
The repackaging operation entailed removing the cardboard resin containers from their drum, introducing the containers into the glove box line, and emptying the containers into a suitable mixing pan for inspection and sampling. Although there was rarely any detectable free liquid in the resin package, sufficient moisture was present to permit the determination of the resin pH. A 50 wt% solution of sodium hydroxide was added to the resin to ensure that the pH was a minimum of 7.0. The neutralized resin was then repackaged into 4-liter plastic bottles that contained a maximum of 2 kilograms of resin or 12 grams of plutonium. The repackaged resin was removed from the glove box line, assayed to determine the exact plutonium content, and placed back into a 55-gallon drum awaiting shipment to the Bottle Box.
Building 774, which housed the Bottle Box, had a comparatively low limit on the amount of plutonium that could be stored in the building at any given time. Therefore, the shipment of drums of repackaged resin was accomplished on a just-in-time basis ensuring an uninterrupted flow of resin to the Bottle Box while not exceeding building plutonium limits.
The actual cementation operation is taking place as previously described. One bottle of repackaged resin and up to sixteen bottles of aqueous liquid waste are introduced to the Bottle Box. The liquids are collected in a feed tank, mixed, and neutralized. The liquids are then drained from the feed tank into the drum of dry cement that was previously prepared. At this point, a single bottle of resin is also added to the drum. The cement is then mixed by hand and allowed to cure. The drum is then disengaged from the glove box for storage and ultimate disposal. The maximum throughput of the Bottle Box operation is two batches per week.
A flow chart of the combined resin repackaging and Bottle Box operation is shown in Figure 2. As of October 1997, approximately one-third of the residue ion exchange resins has been stabilized in this manner. Over 60 Bottle Box runs have been completed each of which has included between 1 and 2 kilograms of resin. The operation will continue in this manner for at least one more year after which all of the residue ion exchange resins will have been incorporated into a cement monolith. Completion of this activity will result not only in the stabilization of a possibly unstable residue of particular concern to the DNFSB, but also the complete elimination of an entire inventory of one type of residue at Rocky Flats.
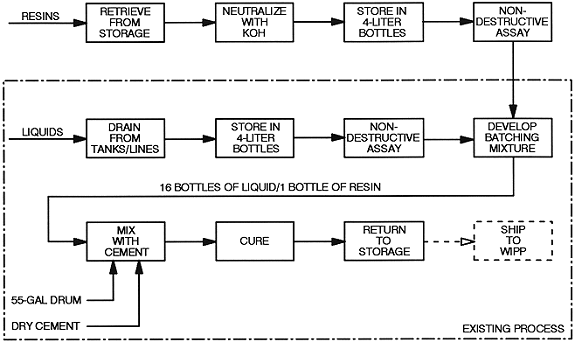
Figure 2. Treatment of Residue Ion Exchange Resins
CONCLUSIONS
Rocky Flats has undertaken a program to stabilize residue ion exchange resins by cementing the resins in conjunction with the cementation of aqueous liquid wastes. This method of treatment was determined to be the most expeditious and cost-effective means to address a safety concern raised by the DNFSB. By virtue of a provision in the WIPP waste form requirements, the cooperative treatment of two waste streams will result in a reduction in the total number of drums of TRU waste by approximately 180. Currently, the program is about one-third complete. The reduction in the amount of effort to commission a new waste treatment facility; the avoidance of having to staff and operate a separate treatment process; the elimination of secondary liquid wastes; and the reduction in the number of TRU waste drums requiring storage, certification, and disposal will result in an eventual cost savings to the Site of nearly 1 million dollars.
REFERENCES