A STUDY OF PHYSICOCHEMICAL PROCESSES DETERMINING THE
STATE OF WASTE COMPONENTS IN
UNDERGROUND REPOSITORIES
I. M. Kosareva and M. K. Savushkina
Institute of Physical Chemistry
Russian Academy of Sciences
Moscow, Russia
ABSTRACT
High-level radioactive wastes (HLW) having a complex macrocomposition and containing radionuclides with various half-lives require particular attention during the disposal of liquid radioactive wastes into underground repositories. Significant changes occur in the composition of wastes under the specific conditions of an underground repository. These changes largely determine the behaviour of the radionuclides present in HLW.
The study of the thermal hydrolysis and related redistribution of radionuclides between liquid and solid phases has been carried out on HLW imitators. New data on radiation transformations in nitrate-acetate systems have been obtained. These data allow us to predict the transformation of waste compositions in various parts of an underground repository.
A long-term prediction of the state of waste components in underground repositories is a topical problem for the practice of underground disposal of liquid radioactive wastes. For the mathematical simulation of the migration of radioactive waste components to be carried out, it is necessary to know and to take into account new hydrogeological conditions that arise in individual parts of an underground repository at different periods of time. The main parameters of new geochemical equilibria will be determined by a complex of physicochemical transformations that occur in the repository under the technogeneous influence of wastes. The assessment of the waste compositions and the study of the results of many years' observations of the distribution of wastes in the porous confinement layer allowed us to establish the main physicochemical processes responsible for the behaviour of waste components. These processes and the results of laboratory experiments on their study were described in detail in out earlier paper [1].
It was shown that when subjected to specific thermal treatment high-level wastes (HLW) become heterogeneous, which may considerably influence the state of radionuclides present in them, However, the extent of their redistribution between the liquid and solid phases depends on many factors. Thus, the macrocomposition of the liquid phase, specifically the concentration of nitrate and acetate ions and the pH value determine the state of Ce-144: the amount of Ce-144 that passes to the solid phase may change from few to 99.9% of the initial content.
Another process characteristic of HLW is the radiolytical transformation of the liquid phase. As was shown in [1], nitrate and acetate ions are subject to radiolysis. The permanent toxicological threat that nitrate ions present makes this process very significant for the assessment of the safety of the method.
This paper presents new results of the study of the thermal hydrolysis and radiolysis of HLW, including the behaviour of macrocomponents and long-lived fission products. The intent of the work was not only to study these processes but also to reveal the characteristic parameters and relationships that can be used in the mathematical simulation of the migration of individual components of wastes.
The study was carried out on systems with [Fe] ~ 0.05 mol/l, [Cr] ~ 0.025 mol/l, [Ni] ~ 0.02 mol/l, [Mn] ~ 0.02 mol/l, [Ac] ~ 0.6 mol/l , and [NaNO3] ~ 2 mol/l. Subjected to thermal treatment (t=80 - 95o) were systems with pH 2-6. The establishment of a new equilibrium state was determined from the analysis of the liquid phase for all the cations present (except Na+) and acetate ions. The cationic analysis was carried out using atomic absorption (Karl Zeis AAS-1 spectrometer). The instrumental sensitivity was 2.0 mg/l for Fe and 2.5 mg/l for Cr, Mn, and Ni. Acetate ions were determined using a modified method of acid-base titration.
The composition of the solid phase was determined from the results of analysis of the filtrates obtained during pH determinations under the condition of a heterogeneous equilibrium.
The behaviour of radionuclides (Ce-144, Sr-90, and Cs-137) under precipitation conditions was studied using the radiometric method as the most reliable and sensitive method for low concentrations (RFT 20026 analyser (Germany), scintillation plastic or KI crystal).
Table I shows the experimental data on the composition of the resulting solid phases as a function of the initial pH of the system. As follows from Table I, the precipitates do not contain nickel and manganese cations; i.e., these cations remain in solution. At pH 6.5, the solid phase contains traces of manganese. Acetate ions are found in the solid phase over the whole pH range. Iron is fully hydrolysed at pH 1.8-2.0. The precipitation of chromium is spasmodic: at pH 3.0-3.5, chromium cations are still in the liquid phase, while at pH 4.0-4.5, chromium is already 80% hydrolysed. A further increase in pH up to 6.0-6.5 is accompanied by a full precipitation of iron and chromium ions, and only manganese and nickel cations remain in solution.
Table I. Influence of Temperature on the Composition of Precipitates
Forming
During Thermal Hydrolysis (t = 80-95° C)

The assessment of the redistribution of radionuclides between the liquid phase and newly formed solid phase is important for the study of the dehomogenization of the systems in question.
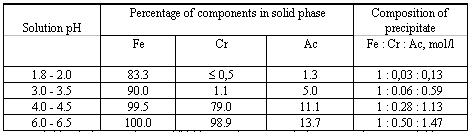
Figure 1 shows the data characterising the behaviour of fission products during the thermal hydrolysis of the solutions of the above-mentioned composition as a function acid-basic parameters. According to the results obtained (Fig. 1), acid-basic properties of the liquid phase have the strongest effect on the behaviour of Ce-144. At the beginning of precipitation (pH 2-3), the degree of the Ce-144 precipitation is not more than 10-15% of the initial concentration. The amount of the solid phase increases and its composition changes with an increase in pH, which is accompanied by a considerable increase in the Ce-144 concentration in the precipitate. At pH 5.5-6.0, almost all Ce-144 passes to the solid phase.
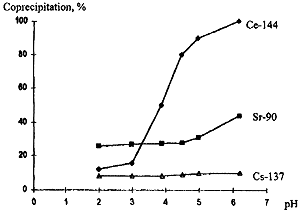
Figure 1. Behaviour of Radionuclides During Thermal Hydrolysis
The results obtained correlate well with changes in the form of existence of Ce3+ species in acetate solutions with different pH: the cationic form [Ce3+] prevails at pH 2-3, while cerium acetate complexes [CeAcn]3-n dominate at pH 2-3. The high degree of the Ce-144 precipitation at pH 5-6 (Fig. 1) suggests that cerium is involved in the formation of acetate compounds.
The acid-basic parameters of the system have a much weaker effect on the behaviour of other fission products: Sr-90 and Cs-137. Thus, at pH 2.0-5.0, the coprecipitation of Sr-90 accounts for 20% of the initial concentration and only at pH~6.0, it increases to about 50% of the initial concentration. Almost all (about 90%) Cs-137 remains in the solution over the whole pH range studied.
Figure 2 shows the data on the effect of the macrocomposition of the liquid phase on the degree of Sr-90 coprecipitation. At pH 2.0-4.5 (Fig. 2, curve 1), Sr-90 coprecipitation is constant over the whole concentration range. The salt background (NaNO3) hardly has any effect on the process (Fig. 2, curve 2). An increase in pH to 6.0-6.5 contributes to Sr-90 transition to the solid phase. In this range, the degree of precipitation is more dependent on the Sr-90 concentration in the solution and the total salt concentration (Fig. 2, curves 3 and 4).
Figure 2. Percentage of Sr-90 Coprecipitation as a Function of Concentration and Macrocomposition of the Liquid Phase
The experimental data obtained are well described by the linear equation
S = -alog[c] + b, |
(1) |
where S is the radionuclide concentrations in the solid phase (%); [c] is the initial radionuclide concentration in the solution (mol/l); and a and b are coefficients.
Table II shows a and b coefficients for solutions with different parameters. The results of the analyses allow us to suggest the mechanism of the capture of Sr-90 by the solid phase.
Table II. Coefficients for Calculation of the Coprecipitation of Radionuclides
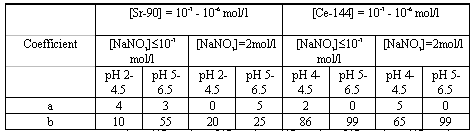
The absence of the competitive effect of the Na+ macrocomponent on the degree of Sr-90 coprecipitation at pH 2.0-4.5 indicates that, under these conditions, Sr-90 is not involved in the formation of the solid phase and that a physical soprtion of its microquantities on the precipitate takes place.
In passing to higher pH values, the behaviour of Sr-90 changes: the coprecipitation degree increases, but the negative effect of sodium cations becomes more pronounced, which suggests an ion-exchange character of the process.
Figure 3 shows the degree of Ce-144 precipitation as a function of Ce-144 concentration in the solution for various macrocompositions of the liquid phase. All compositions studied are characterised by a high percentage of Ce-144 transition to the solid phase. At pH 6.0-6.5, cerium is virtually fully localised in the solid phase, whatever the salt background and concentration.
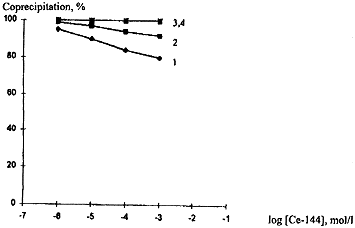
Figure 3. Percentage of Ce-144 Coprecipitation as a Function of Concentration and Macrocomposition of the Liquid Phase
The negative effect of salts on Ce-144 precipitation shows up at lower pH 4.0-4.5 in the range of the highest Ce-144 concentrations. The curve of Ce-144 precipitation as a function of its concentration is described well by equation (1), whose coefficients for different parameters of the liquid phase are given in Table II.
The behaviour of another long-lived fission product Cs-137 was studied by an analogous pattern. The obtained data showed that the degree of Cs-137 precipitation is during thermal hydrolysis is never higher than 10%, whatever the composition of the liquid phase and the Cs-137 concentration.
Thermal conditions of underground repositories can considerably influence the behaviour of the macrocations (corrosion products), whereas radiation fields have a determining effect on the concentration of the major anions of HLW: nitrates and acetates [1].
A study of the radiolytical processes occurring in HLW is a topical problem, whose solution will serve to ensure the safety of HLW processing, including both underground repositories and special tanks. The study of the radiolytic transformations of nitrate-acetate systems was carried out in the range of high absorbed doses 0.25 - 2.0 MGy characteristic of a long-term storage of HLW. Series of solutions containing (0.1 - 0. 5) M HNO3 and (0.25 - 1.0) M CH3COOH were selected for irradiation. g -Irradiation was carried out with a 60Co source at a dose rate of 25 and 16 kGy/h, respectively. The parameters studied were the acidity of solutions and the concentrations of nitrate and acetate ions.
The observed (not initial) radiation-chemical yields were determined as follows. The decomposition curves were normalised to the concentration of the component measured at the dose D = 0. The normalised curves were approximated with second-order polynomials Y = a + bD + cD2, and the derivative dY/dD/ D=0 = b and the radiation yield G = b*n*Co were calculated, where Co is the initial concentration of the component and n is the dimensionality coefficient. The overall error of the yield determination did not exceed ±20%. The method of the normalisation of the dose/effect curve was also used in the analysis of the material balance of the radiolytic decomposition of acids in the systems studied. This allowed the experimental material to be studied as an integral whole [2].
Figure 4 shows the results of the kinetic study of the radiolysis of the systems. The X axis shows the normalised sum of the concentrations of acetate and nitrate ions, measured by direct methods, and the Y axis shows the normalised sum of the concentrations of hydrogen ions, determined by potentiometric titration in samples irradiated with different radiation doses. Figure 4 is thus a generalised graphical representation of the material and electrical equilibria during radiolysis. The straight line is calculated by the least-squares method Y = 0.951X; the correlation coefficient is equal to 0.989. The data shown in Fig. 4 show that the overall acidity [H+] = [HNO3] + [CH3COOH] decreases during g -irradiation of nitrate-acetate solutions. A study of the experimental data showed that the acidity was determined only by the sum of the concentrations of nitric and acetic acids, whereas the acid products of the radiolysis of acetic acid do not contribute to the acidity. This means that the effect of the complex formation with oxy- and diacids (the products of CH3COOH radiolysis [3]) on the homogeneous stability of HLW can be excluded.
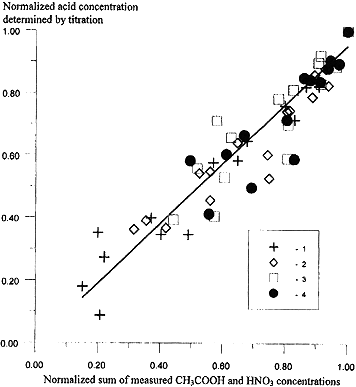
Figure 4. Material Balance During Radiolysis of a Simulator of High-level Radioactive Waste Subject to Disposal. Composition: 0.3 M HNO3 in the presence of 0.25 (1), 0.5 (2), 0.75 (3), and 1.0 M CH3COOH (4)
Table III shows the dependence of the yield of decomposed acetic acid on its initial concentration. As follows from the table, there is no single dependence of G(-CH3COOH) on the initial CH3COOH concentration in the studied range of HNO3 concentrations. Each acetic acid concentration has its own dependence of G(-CH3COOH) on [CH3COOH].
Table III. Decomposition Yield of CH3COOH in Nitrate-acetate Systems

The assessment of the yield of the acidity change [G(-H+), ion/100 eV] in these systems showed that, within the experimental error, this value does not depend on the initial CH3COOH concentration and is equal to G(-H+) = 2.84 ± 7.6 ion/100 eV with a confidence coefficient of 0.95.
Thus, the study of the radiolysis of the HNO3-HAc system did not reveal the formation of additional acid products with chelate properties. The obtained radiation-chemical yields of the change in acidity can be used for the prediction of new acid-base properties of HLW as a function of the time of localisation in the underground repository.
Summing up the data presented, one can conclude that the physicochemical transformations occurring in underground repositories lead to significant changes in the composition and properties of high-level liquid radioactive wastes. Therefore, a long-term prediction of the migration of wastes in an underground confinement layer requires a comparatively large mass of experimental data on the form of existence of waste components in individual parts of an underground repository.
REFERENCES