THE STUDY ON RADIOLYTIC GAS EVOLUTION FROM
NITRATE-ACETATE AQUEOUS SOLUTIONS
MODELLING RADIOACTIVE WASTES
A.K.Pikaev, G.N.Pirogova, I.M.Kosareva, M.K.Savushkina and S.A.Kabakchi
Institute of Physical Chemistry of Russian Academy of Sciences
Leninsky Prospect, 31
Moscow 117915, Russia
ABSTRACT
The report summarizes the results obtained from the study on radiolytic gas evolution from nitrate-acetate aqueous solutions modelling liquid intermediate- and high-level radioactive wastes stored in special tanks and disposed to underground repositories. The volume and composition of gases evolved upon g -radiolysis of these aqueous solutions were determined. It was found that the origin of gases evolved depends on the composition of the solutions. The gases detected were H2, O2, CH4, N2, CO2 and N2O. Methane and carbon dioxide are formed in solutions containing sodium acetate or acetic acid, N2O appears in acid solutions in the presence of nitrate and acetate ions. The radiation-chemical yields (G-values) of gases were measured under various experimental conditions. In some cases, the data obtained with model solutions were compared with the results from the study on real liguid radioactive wastes.
INTRODUCTION
To present time, a large amount of liquid radioactive wastes was accumulated as a result of processing nuclear materials [1-3]. They are stored in special tanks or deep underground repositories. The wastes contain nitrates, nitric acid, acetates, acetic acid, carbonates, alkali, metal corrosion products, some organic admixtures. The main components of all the wastes in Russia are nitrate and acetate ions. The wastes are acid and alkaline.
The wastes can be highly-radioactive (up to several hundred curies per litre at initial stage of storage in tanks and up to several curies per litre just before disposal to deep underground repositories). Because of it radiolysis of wastes occurs. It is accompanied by gas evolution. Among the gases can be H2 and CH4 which are explosive under some conditions. Besides, upon underground storage of wastes, the formation of gases can give rise to the disturbance of penetrability of rocks, to the effects on moving the wastes in the repository and so on. Hence, for successful radioactive waste management, it is necessary to know the features of radiolytic gas evolution from these systems.
There are a lot of publications (see books [4-6] for references) devoted to radiolytic conversions of nitrate solutions including the formation of gases (predominantly H2). There are some information (see, e.g., book [6]) on radiolysis of acetic acid aqueous solutions. However, the data on gas evolution from nitrate-acetate solutions upon radiolysis are virtually absent. Taking it into account, we performed the detailed investigation of this process. Some results obtained were published earlier [7,8].
EXPERIMENTAL
A 60Co g -ray source was used. Dose rate was equal to 10-12 kGy/h. Dosimetry was performed with dixpomate dosimeter [9].
A special glass reactor with free volume was utilized in the experiments. It was connected with a check pressure gauge in order to determine the amount of gas formed upon g -radiolysis by measuring a pressure increment. The reactor was equipped with a thermostated jacket to maintain a constant temperature. As a rule, temperature was equal to 30 ± 1° C. Before irradiation, the solution was deaerated by bubbling helium for one hour.
The gases evolved were determined by the following procedure. After irradiation to a given dose, increase in the pressure value in free volume of the reactor was measured for the determination of total amount of gas evolved as a result of irradiation. Gas samples were taken with a syringe and were introduced into a LKhM-8 MD chromatograph (helium as a carrier gas, katharometer as a detector, and columns packed with Porapack-Q or molecular sieves 5A). The sensitivity was 10-3 vol %; the relative error was ± 10%. Using the analytical data obtained, radiation-chemical yields (G-values) were calculated. The error of the determination of the yields was not more than 20% at confidence level 0.95.
RESULTS AND DISCUSSION
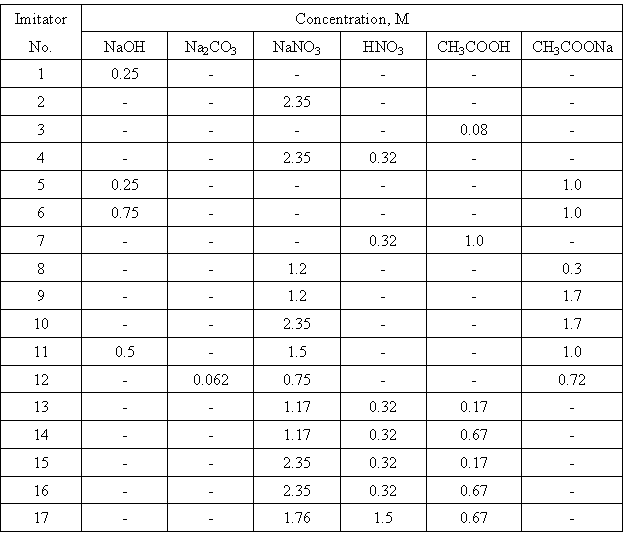
The composition of studied model solutions (waste imitators) is presented in Table I. As an example, Fig. 1 shows dependences of total amounts of gases evolved from acid nitrate-acetate imitators on absorbed dose.
Table I. Composition of Imitators

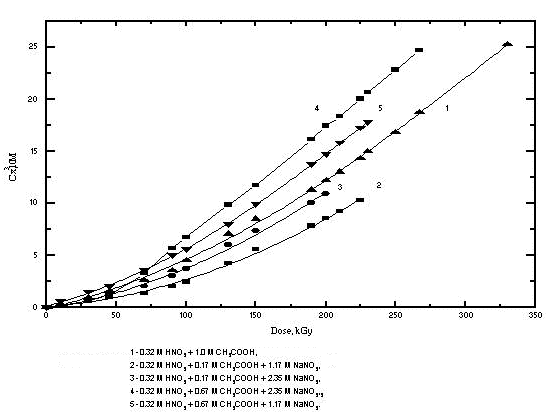
Figure 1. Dose Dependence for the Amount of Evolved Gas from Radiolysis of Solutions: 1 - 0.32 M HNO3 + 1.0 M CH3COOH, 2 - 0.32 M HNO3 + 0.17 M CH3COOH + 1.17 M NaNO3, 3 - 0.32 M HNO3 + 0.17 M CH3COOH + 2.35 M NaNO3, 4 - 0.32 M HNO3 + 0.67 M CH3COOH + 2.35 M NaNO3, 5 - 0.32 M HNO3 + 0.67 M CH3COOH + 1.17 M NaNO3.
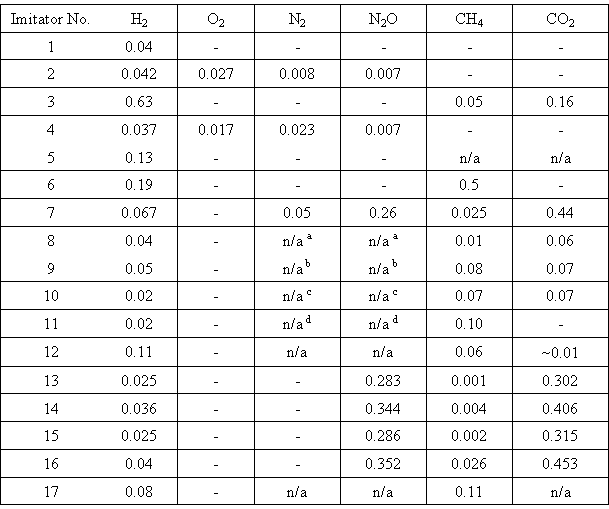
From the Figure, two main features are seen. The first consists in the fact that in each system, there is some "slown-down" period in gas evolution at low doses. Obviously, it is caused by saturation of imitators with the evolved gases. At higher doses, the total amount of gases depends on dose more strongly. Radiation-chemical yields of each gas were determined from the second parts of the curves similar to those shown in Fig.1. The values obtained are presented in Table II.
Table II. Radiation-chemical Yields of Gases from Waste Imitators (molecule/100 eV)
a
In the Table "n/a" means "not analysed". The total yield of nitrogen-containing gases was equalThe second feature is the higher amount of evolved gases in nitrate-acetate system than in the imitator containing only nitrate ions. The increase in nitrate concentration slightly affects the gas evolution, but in the presence of acetic acid, twofold increase in the yield is observed.
As it follows from Table II, the origin of gases depends on the composition of the solution. In the solution of NaOH, only H2 was recorded. Gases appeared in CH3COOH solution are H2, CO2 and CH4. In alkaline solutions of CH3COONa, H2 and CH4 are evolved into gas phase; CO2 reacting with alkali forms carbonate. Radiolysis of solutions of NaNO3 or NaNO3 and HNO3 leads to the formation of H2, O2, N2 and N2O, but two last gases are formed with very low radiation-chemical yields. The addition of acetate ions to acid nitrate solutions give rise to the considerable increase in G(N2O); however, in neutral and alkaline nitrate-acetate systems, this effect is almost absent. Note that the influence of organic additives and acidity of the solution on G(N2O) was also described in paper [10].
The evolution of hydrogen was also measured from real high-level radioactive waste stored in stainless steel tank. The waste was a solution of the composition (mol/dm3): NaNO3 1.75, HNO3 1.9 and CH3COOH 0.67. The measured values of G(H2) after 10, 30 and 66 days of storage of the waste with initial specific activity of about 660 Ci/dm3 were equal to 0.04, 0.05 and 0.04 molecule/100 eV.
The data obtained allow us to propose that in waste imitators there are several sources of radiolytic gases. The first of them is the formation of molecular hydrogen in spurs. The decrease in G(H2) with increasing nitrate concentration is due to scavenging hydrated electrons in spurs by nitrate ions at their high concentrations.
The second source are the reactions of solutes with primary products of water radiolysis. One of the most important reactions is the interaction of H atoms with acetate ions:
|
|
(1) |
explaining the increase in G(H2) values in the solutions containing these ions.
The formation of nitrogen-containing gases can be connected with the conversions of NO32- and NO2 radicals formed in reactions:
|
|
(2) |
|
|
(3) |
In acid medium, NO32- is converted to NO2:
|
|
(4) |
At high doses, NO2 and/or its dimer N2O4 can compete with nitrate for eaq and H:
|
|
(5) |
|
|
The further reactions of NO and N2O3 leading to the formation of N2 and N2O can be as follows:
|
|
(7) |
|
|
(8) |
|
|
(9) |
|
|
(10) |
|
|
(11) |
Acetate ions suppress back oxidation of NO2 or NO2- by OH radicals:
|
|
(12) |
|
|
(13) |
The role of hydrogen ions seems to consist in the stabilization of nitrogen oxides.
The third source is the direct action of ionizing radiation on the solute. This effect is caused by the fact that wastes and their imitators are concentrated solutions. Apparently, it is responsible for the formation of methane and CO2 (partially) from acetate and oxygen from nitrate:
|
|
(14) |
|
|
(15) |
|
|
(16) |
|
|
(17) |
The fourth source can be interaction of radiolysis products with each other. This source seems to be responsible for the formation of the part of CO2 because G(CO2) > G(CH4). It is not excluded that acetaldehyde which is a product of radiolysis of acetic acid can react with some other radiolysis products forming carbon dioxide. It is not excluded that other pathways for the formation of radiolytic gases (especially nitrogen-containing ones) can exist.
The data obtained were used for the calculations of amounts of air which should be blown over the waste in tank in order to prevent the formation of explosive mixture of hydrogen and methane with oxygen. Besides, the forecast of radiolytic gas evolution from the wastes in underground repository is under development.
REFERENCES