
STABILIZATION OF LIQUID PLUTONIUM AND URANIUM
RESIDUES AT THE ROCKY FLATS ENVIRONMENTAL
TECHNOLOGY SITE
W. A. Franz and R. S. Schmidt
Safe Sites of Colorado, L.L.C.
ABSTRACT
Following termination of operations, Rocky Flats Environmental Technology Site (RFETS) retained about 29,000 liters of solution containing levels of actinides ranging in concentration from less than 1 gram/liter to 310 gram/liter. These solutions resided in bottles, tanks, and piping. Much of the volume was acid up to 10 N and posed a significant hazard in case of a leak.
RFETS, in collaboration with Los Alamos National Laboratory, developed a strategic approach to stabilizing the solutions, combining several techniques. These techniques could be applied to other actinide solutions. The approach to draining tanks and piping utilized vacuum transfer to critically safe 4 liter bottles, which could be transferred to one of several processes. For some piping systems, a tapping device was installed at low points and the system drained to a specially-designed portable system. Stabilization processes included direct cementation of low concentration solutions, beaker scale precipitation and filtration of uranium and plutonium chloride solutions, and automated precipitation and filtration of plutonium nitrate solutions. The automated precipitation utilized the Caustic Waste Treatment System, which allowed processing of solutions up to 150 gram/liter, resulting in filtrate of 10
-4 gram/liter.RFETS expects to have drained and processed all actinide solutions by July 1999. This demonstrates that a small number of techniques can be adapted to removing and stabilizing a variety of actinide solutions occurring in widely varying locations.

Figure 1. Rocky Flats Environmental Technology Site
PROBLEM
When production operations were curtailed at Rocky Flats in 1989, approximately 29,000 liters of actinide solutions remained in tanks, piping, and bottles. These container systems were not designed for long term storage, and deterioration had occurred over time. Polyethylene and polypropylene bottles deteriorated and became brittle with long-term radiation exposure. Flanged pipe joints and valves leaked. Some tanks depend on Raschig rings for criticality safety, and no inspection of the rings to verify their effectiveness had been done. Hydrogen generation in tanks and bottles presented a threat of fire or explosion. Because of these problems, removal and stabilization of actinide solutions has been one of the highest priorities in the cleanup of the Rocky Flats Environmental Technology Site.
One of the problems faced by the cleanup team was the diversity of characteristics and locations of the solutions. While most of the volume was plutonium nitrate (Pu(IV)), concentration ranged from <1 gram/liter to 310 gram/liter. Some solution contained uranium (UO
2(NO3)2) or chlorides from hydrochloric acid. Locations included one liter and 4 liter bottles in gloveboxes or packed in drums, annular or Raschig ring tanks, and piping systems, primarily in Buildings 771 and 371. In addition to existing solutions, laboratories on site continued to generate waste solutions, and decontamination generates additional liquid. An approximate inventory of actinide liquids is presented in Figure 2.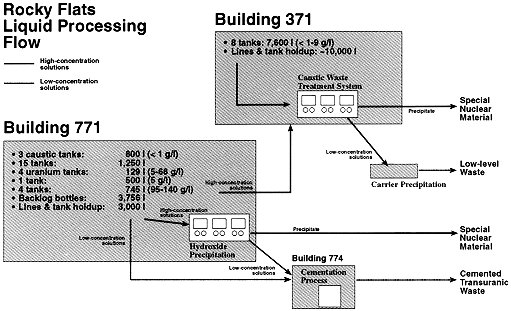
Figure 2. Rocky Flats Liquid Processing Flow
AVAILABLE TOOLS
Rocky Flats considered a number of techniques for liquid stabilization (Ref. 1), and developed a baseline strategy. Precipitation techniques would be used to reduce actinide concentrations to levels acceptable to existing liquid waste management systems, and treatment of liquids would be performed in the two main liquid treatment buildings to minimize transfer. Precipitation protocols were evaluated at Los Alamos National Laboratory (Ref. 2). Based on their testing, a recommendation was made to use Pu(III) oxalate precipitation for high concentration solutions and magnesium hydroxide precipitation for lower concentration solutions. The magnesium hydroxide process was shown to be effective for solutions below 45 gram/liter. In Building 771, oxalate precipitation followed by magnesium hydroxide polishing was selected. Filtrate and solutions already below 1.5 gram/liter would be cemented in the Building 774 "bottle box". In Building 371, which was not expected to contain high concentration solutions, the Caustic Waste Treatment System (CWTS) would treat solutions containing greater than 0.004 gram/liter using magnesium hydroxide. Filtrate and solution already meeting this criteria would be sent to a carrier precipitation system in Building 374. From the baseline plan, Rocky Flats program staff developed a detailed plan with some key modifications designed to simplify and accelerate solution stabilization.
First, a more detailed description of the available tools is needed. Oxalate precipitation, magnesium hydroxide precipitation, "bottle box" operations, and carrier precipitation will be described.

Figure 3a. High-concentration Pu Tanks in Rocky Flats Building 771

Figure 3b. Hydroxide Precipitation Conducted in Glove Boxes at Building 771

Figure 3c. Packaged Precipitate from Building 771 Liquid Stabilization
Oxalate Precipitation
For high concentration solutions in Building 771 tanks, Los Alamos recommended a process of Pu(IV) reduction using hydroxylamine nitrate (HAN) followed by Pu(III) oxalate precipitation. In this process, Pu(IV) is reduced:
4Pu
+4+2NH3OH+®4Pu+3+N2O+H2O+6H+ and2Pu
+4+2NH3OH+®2Pu+3+N2+2H2O+4H+Pu(III) is then precipitated by addition of solid oxalic acid:
2Pu(NO
3)3+3H2C2O4+10H2O®Pu2(C2O4)3 10H2O+6HNO3Any remaining HAN is eliminated by a reverse strike of the solution to a sodium nitrite slurry. This step prevents the formation of ammonium nitrate. The final process step is the calcination of the oxalate:
Pu
2(C2O4)3 . 10H2O+2O2®2PuO2+6CO2+10H2OLos Alamos found that the filtrate did not meet the process requirement. Therefore, an additional precipitation step was required following oxalate. Hydroxide precipitation was recommended.

Figure 4a. Typical Tank Containing Actinide Solutions in Building 371

Figure 4b. Caustic Waste Treatment System Operations in Building 371
Hydroxide Precipitation
For use in beaker scale precipitation of Pu from chloride contaminated solutions and for U solutions, Los Alamos recommended hydroxide precipitation. This was also the recommended process for low concentration (<6 g/l) solutions in the CWTS. Both potassium hydroxide and magnesium hydroxide were tested, but magnesium hydroxide was recommended for several reasons. It could be added as a solid, and filtration time was significantly shorter. Also, magnesium hydroxide generated less volume of precipitate and provided a filtrate lower in actinides. Precipitation is straightforward:
Pu
+4+4OH-+xH2O®Pu(OH)4 . xH2OWater is evaporated to leave Pu oxide.
Uranium solutions can also be precipitated as magnesium uranates:
2UO
2(NO3)2+3Mg(OH)2®MgU2O7+2Mg(NO3)2+3H2O orUO
2(NO3)2+2Mg(OH)2®MgUO4+Mg(NO3)2+2H2OHydroxide precipitation is used in the Caustic Waste Treatment System (CWTS) in Building 371 (see Figure 2). Solutions are collected in 4 shielded annular tanks of nominal 590 liters each. Transfer within Building 371 is by gravity, vacuum assisted. Solution can also be drawn from containers in the CWTS glovebox using a vacuum wand. Solutions can be transferred between tanks to blend for optimum processing parameters. Precipitation occurs in two 30 liter glass columns by addition of solid magnesium hydroxide. Initial solution is <6 gram/liter and <3.5 N acid, and magnesium hydroxide is added to raise pH to above 6. The solution is then filtered in two stages to remove the insoluble plutonium hydroxide. Figure 5 shows typical precipitate. The precipitate is assayed for actinide content and stored. The filtrate is sampled for carrier precipitation acceptance criterion (<0.004 gram/liter) and pumped to storage in Building 374 if the criterion is met. If not, the filtrate is recycled through the precipitation process. Typical filtrate is near 0.0001 gram/liter, and recycle batches have been rare.

Figure 5. Typical Precipitate
Direct Cementation
Direct cementation is performed in a glove box in Building 774 for solutions containing less than 6 gram/liter actinides (Ref. 3). Bottled solutions are transferred into the glove box and vacuum transferred to a mixing tank, where they are adjusted for pH by addition of sodium hydroxide. The solution is then added to a 55 gallon drum connected to the glovebox. The drum contains a mix of portland cement and a proprietary insulating cement. The mixture is stirred and allowed to set, resulting in a TRU waste form. To increase packaging efficiency, a small amount (<1%) of ion exchange resin, another Rocky Flats waste, can be added to the cement.
Carrier Precipitation
Carrier precipitation, located in Building 374, is an existing plant waste water treatment system. It can accept solutions up to 0.004 gram/liter. The process involves pH adjustment to >7 and the addition of ferrous sulfate, calcium chloride, magnesium sulfate, and a flocculating agent to cause precipitation of actinides. The process is cycled until the treated water is below 2 X 10
-7 gram/liter, at which point it can be returned to the plant process water system. The precipitate forms a sludge, which is evaporated to a low level waste form.PROCESS SELECTION
The original approach at Rocky Flats was to process solutions contained in Building 371 or 771 in that building. Bottles stored or generated in other buildings would be transferred to Building 771. Building 771 would use beaker scale hydroxide precipitation or oxalate precipitation for higher concentration solutions and direct cementation for the filtrate or lower concentration solutions. Building 371 would use the CWTS for solutions exceeding the 0.004 gram/liter acceptance criteria of the Building 374 waste water system. This changed early in 1997.
In December 1996, the CWTS began operations processing solutions of up to 6 gram/liter. However, this system was designed to be critically safe to 150 gram/liter and has the flexibility of 4 receiving tanks that can blend solutions to optimize processing parameters. The CWTS quickly proved to be a reliable system with high throughput. Initial plans for 80 liters/day were exceeded as experience was gained. In November 1996, Building 771 began beaker scale processing of uranium and chloride solutions. After some initial difficulty due to the chemistry variations in feed solution, the Building 771 hydroxide precipitation process began operating smoothly as well. As a batch process, the volume rate was much lower than CWTS, but it was proving a good process for low volumes of special solutions. It was apparent that magnesium hydroxide precipitation was a flexible and viable process.
Equipment for oxalate precipitation in Building 771 was still in the design stage. Operations and program personnel decided to evaluate the feasibility of canceling that development and relying on magnesium hydroxide. In addition to avoiding the cost and technical risk of a new process startup, the idea presented significant advantages to the schedule for Rocky Flats cleanup. Building 771 had been rated as the most hazardous building in the DOE complex by the Plutonium Vulnerability Study and was targeted as the first major building to be decommissioned. The liquid residues, especially the high concentration residues, presented the greatest hazard. Because of this hazard, substantial resources were committed to the safety systems and administrative controls required to maintain the safety envelope. Removal of the hazard would improve safety and free resources to accelerate decommissioning. Projected process rates for the oxalate process put the draining and processing duration at 9 months, for a May 1998 completion. If high concentration liquids could be transferred from Building 771 to Building 371 for processing in CWTS, removal could be complete in December 1997. Processing time would not be accelerated, but the high concentration liquids would be in new shielded annular tanks in a welded system, a better configuration than Building 771.
A number of challenges to the proposal existed. First, transfer of the liquids from Building 771 to Building 371 would be required. Site procedures allowed for the transfer of liquids in 4 liter bottles inside 55 gallon drums with up to 200 grams fissionable material per drum. The four high concentration tanks contained about 88 kg of fissionable material at concentrations ranging from 95 to 140 gram/liter. The earlier planning study (Ref. 1) concluded that transfer of this much material under current site procedures would be impractical. Second, although CWTS could receive and store liquid containing up to 150 gram/liter, existing procedure limited processing in the columns to 6 gram/liter. Dilution would be required for processing. Technically, process water could be used, but this would create additional waste water. The program rejected this option. Building 371 did have two sources of diluent, however. One tank, a receiver from the building criticality drain system, was scheduled to be drained and processed. This tank contained approximately 2200 liters of <1 gram/liter solution. Also, room decontamination using high pressure water spray was underway, which generated about 2000 liters per room. These large volumes of low concentration liquid drained to the CWTS. The final challenge was authorization basis for the operation. This is the technical safety basis upon which permission to operate is based, the "license". At Rocky Flats, each building has a separate authorization basis, most based on the production mission. The authorization bases address safety of the public and of workers on site but not in the facility (co-located workers), not workers involved in the process. The issues of new mission, coordination between buildings, and process workers were addressed through a number of the infrastructures (Criticality Engineering, Nuclear Safety, etc.), but they were brought together in an integrated safety document called an Activity Control Envelope. This is the Rocky Flats vehicle for integrated safety planning.
Based on an assessment of the benefits and risks, the new approach was approved in February 1997. Work was stopped on oxalate precipitation in Building 771, and the alternate plan to transfer high concentration solutions to Building 371 for processing was initiated. Figure 2 shows this integrated approach. Because its solutions are of relatively low concentration and are piped to the CWTS, with the exception of line draining, Building 371 is of interest primarily as the treatment facility. This narrative will follow the path of various solutions from Building 771 through disposition.
All tank draining in Building 771 was performed by vacuum transfer to a small reservoir in a glovebox, which could then be drained manually to fill bottles to a precise volume. Vacuum, rather than pump transfer, was used to minimize the consequences of a leak in the system. Tanks containing solution of up to 140 gram/liter were drained using this method, requiring stringent safety precautions. The two primary hazards of the solution were leaks and criticality. Any leak would result in serious radiological contamination of the immediate area, including airborne contamination. Direct leak testing of the piping was difficult, due to congestion and contamination in the areas of pipe runs. A gross check was conducted by placing the appropriate system under vacuum and listening for in leakage using an ultrasonic probe. Flanged joints were tightened prior to use. Plutonium facility design, with zones of decreasing pressure from uncontaminated to highly contaminated areas, would mitigate the consequences of any leak to the immediate area. Criticality safety was maintained by geometry whenever possible. Any area which could contain a spill, such as a room berm or a glovebox, is designed to limit depth to 2" or less. The reservoir used as a plenum for filling bottles is a cylinder 5" in diameter. Bottles used to collect solution are 4 liters in volume, normally filled to 3.75 liters. The one administrative control used was in bottle handling and staging. Filled bottles were staged in spacer racks to maintain a minimum separation between bottles. Handling the bottles for staging or transfer was controlled administratively to one bottle containing solution out of a rack at one time. Calculations showed that two bottles were critically safe in any configuration, but three bottles would exceed safety margins. The "one bottle at one time" restriction provided a contingency against exceeding any margin.
From Figure 2, 24 tanks containing a total of 5500 liters of low concentration were drained in Building 771. In addition, 576 4 liter bottles existed in backlog. This solution was cemented in the Building 774 bottle box, described earlier. This resulted in 141 drums of transuranic waste meeting the WIPP waste acceptance criteria.
A total of 306 liters from 4 tanks and 56 backlog bottles was treated in 2 liter beakers by hydroxide precipitation. These solutions contained uranium at concentrations up to 310 gram/liter or plutonium at concentrations up to 112 gram/liter with greater than 1 ppm chlorides. These solutions were outside the acceptance criteria for the CWTS, and the relatively small quantity made small batch treatment attractive. The filtrate, containing less than 0.1 gram/liter actinide, was cemented in the bottle box, generating 8 drums of TRU. The precipitate was calcined on a hot plate at >500
° C, yielding SNM quantities of magnesium uranate or plutonium oxide. These were assayed by gamma spectroscopy and stored for offsite shipment.Five tanks were slated for transfer to CWTS. The first contained about 500 liters of 5 gram/liter plutonium nitrate. This solution could be accepted at the bottle box, but the gram limit per drum would have resulted in the generation of over 200 drums of waste. In addition, the 5 gram/liter solution could be handled under current site procedures, without the special precautions required of the high concentration solution. The solution was already within the gram limits for CWTS processing, but it was about 7 N and would require blending to meet the 3.5 N processing specification. It therefore served as a good practice run for transfer, receipt, blending, and processing of solution from Building 771 in the Building 371 CWTS. The tank was drained, transferred, and processed in CWTS in June 1997.
The four remaining tanks were the primary challenge. These contained from 95 to 140 gram/liter plutonium in 745 liters of solution. Criticality was an important hazard with solutions of this concentration. Control and separation of the bottles to maintain a safe array was discussed earlier. Transfer of the bottled solutions to Building 371, a half mile from Building 771, posed an additional challenge. No currently certified container was available to move the liquid. FL-10 containers used to move highly enriched uranyl nitrate from Rocky Flats to Irwin, TN were available and certified for uranium, but not for plutonium. These particular containers were not designed for contained transfer, and were therefore not useful for transfer of plutonium solutions, which present a severe airborne contamination hazard. A solution was devised by simply installing a light tube in the center of a 55 gallon drum with annular plates at either end. Two 4 liter bottles could be placed one on top of the other in each drum. The center tube retained the bottles, but maintenance of the center position was not required for criticality safety. This simply provided a physical means to prevent placement of more than two bottles in a drum. Analysis showed that a single planar array of drums containing two bottles of solution each, was critically safe, regardless of the position of the bottles in the drums. Since the integrity of the drums themselves had been verified previously, no testing was required to demonstrate the safety of this package. Note that this is not a DOT package and was approved only for the short building to building transfer under strict controls.
The transfers were completed without incident in December 1997. Processing is underway, and the CWTS staff initiated an additional processing improvement. Based on the Los Alamos results, the hydroxide precipitation process should be viable up to 45 gram/liter plutonium. CWTS verified that the system could process 24 gram/liter solution, a significant improvement over the 6 gram/liter originally planned. Requiring only a quarter of the dilution for processing significantly improved the schedule and further reduced cost.
To summarize, Rocky Flats workers have made significant progress in reducing the hazards left in the plant following termination of production operations. Using relatively simple techniques developed in cooperation with Los Alamos National Laboratory, most of the hazard of actinide solutions has been eliminated. This has allowed accelerated decontamination and decommissioning planning for Building 771. Use of a few techniques has resulted in substantial cost savings (over $1M) and reduced the technical risk of new process development.
REFERENCES