PINHOLE CORROSION OF CH-TRU WASTE CONTAINERS
BY VOLATILE ORGANIC COMPOUNDS
D. P. Zeek
Lockheed Martin Idaho Technologies Company
Idaho National Engineering and Environmental Laboratory
ABSTRACT
In the spring of 1996 at the Idaho National Engineering and Environmental Laboratory Radioactive Waste Management Complex, an epidemic of corroded CH-TRU waste drums was encountered. The observed corrosion was in the form of rusty brown streaks that emanated from pinholes in about the upper one-third of the 55-gal drums. Wet streaks were tested as highly acidic by litmus paper. The liquid that emanated from the pinholes was found to be hydrochloric (HCl) acid. An investigation concluded that the pinholes were localized pitting corrosion caused by HCl acid formed in the drum headspace from reactions involving chlorinated volatile organic compounds (VOCs) in the waste and the unlined steel of the internal drum wall. The pinholes occurred in the upper parts of the drums because this corresponds to the internal headspace region above the rigid liner.
Affected drums had a few to hundreds of pinholes with no detectable release of radioactivity. This was due to the internal packaging of waste in heavy polyethylene and/or polyvinyl chloride waste bags inside a rigid high-density polyethylene liner. The corrective action taken was to overpack pinhole corrosion drums into polyethylene-lined 83-gal drums and to test hundreds of drums with drum filters, but without pinhole corrosion, for the presence of HCl acid in the headspace gas with colorimetric tubes fitted to the drum filters. These colorimetric tubes contain a substance that changes color in reaction to HCl acid when headspace gas is drawn by a hand pump. Only drums that had a significant probability for the presence of HCl acid in the headspace were segregated in storage to allow ready inspection and efficient handling, if needed. It is recommended that any facility involved in the long-term storage of waste or other contents, that include chlorinated VOCs in unlined steel containers, be wary for the possible development of pinhole corrosion.
INTRODUCTION
On March 11, 1996 at the Idaho National Engineering and Environmental Laboratory (INEEL) Radioactive Waste Management Complex (RWMC), drums with small holes and some leaking substance were discovered. These were found during routine operations of moving contact-handled transuranic (CH-TRU) waste drums from storage in air support buildings to storage in Resource Conservation and Recovery Act (RCRA) Type II-permitted storage modules. The programmatic significance of this corrosion phenomenon was two-fold. Firstly, such a phenomenon was unexpected and raised questions about the knowledge of the waste contents. Secondly, holes through the 55-gal drums raised concerns about the possibility for release of radioactivity.
Management and radiation control personnel were alerted. No radiation was detected from any of these drums. The leaking substance, while still wet, exhibited an acidic pH by litmus paper. The vast majority of drums with holes or holes and leaks contained waste with item description code (IDC, sometimes called 'content code') -3. These are solidified organic wastes such as degreasing agents, lathe coolant and hydraulic oil. These events lead to an investigation to identify the failure mechanism involved and any corrective actions.1
PURPOSE
This report identifies the cause and corrective actions for the pinhole corrosion observed on CH-TRU waste drums stored at the INEEL RWMC, and promulgates the lesson learned.
SCOPE
The scope of the investigation included: a database search; visual, nondestructive and chemical examinations; calculations and analyses. The results from these investigations are presented along with the conclusions. In addition, the corrective actions taken and the lesson learned are identified.
DATABASE SEARCH
The databases searched contained information from: waste generators, drum venting, container inspections and the headspace gas sampling program. Limitations of these databases are explored and results from the correlation analysis are presented.
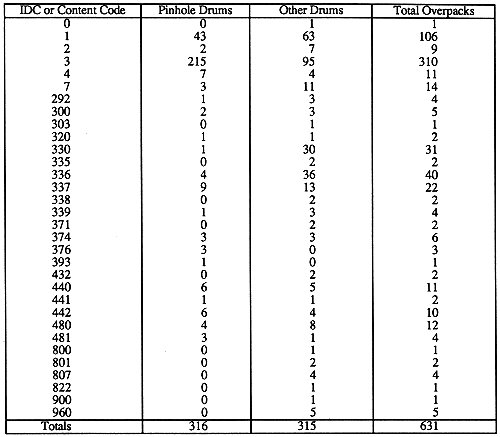
There is some fallability in the container inspection database comment field concerning the reason the drum was overpacked. The operators' comments were not always consistent for a certain type of defect such as pinhole corrosion. For instance, comments such as 'acid,' 'acid drum,' 'drum has pin holes,' and 'possible acid,' were all used for pinhole corrosion drums. The references to 'acid' were used after the leaking fluid was determined to be acidic by litmus paper. It is not known whether pinhole corrosion drums were seen or were overpacked with other ambiguous comments such as 'rust,' before March 11, 1996. With the present awareness, the operators have been sensitized to the characteristics of pinhole corrosion and later container inspection comments included words such as 'pinhole corrosion.' The comment 'liquid between liner and drum' was entered for a drum based on the x-ray real-time radiography (RTR) examination. When these drums went through the container inspection system they were automatically flagged for overpacking and the operator was not allowed to enter a comment on the container condition. At the time, at least 76 content code-3 drums were overpacked with this comment. It is highly probable that a fair percentage of these drums have pinhole corrosion, but they were not able to be counted as such. The actual number of pinhole drums is not known because of the aforementioned variation in container inspection comments. The number of pinhole corrosion drums in Table I was determined by counting drums in the database with either 'acid' or 'hole' in their comment field. These numbers should be considered as a lower bound for the number of pinhole drums.
Table I. Summary of Drums Overpacked from March 11 through May 17, 1996

Information from the databases was examined to determine if there were any correlations to the overpacked pinhole corrosion drums. The overwhelming majority of pinhole corrosion drums overpacked contain organic setups, designated as content code-3 or IDC-003. The only other significant number of pinhole corrosion drums was for content code-1, the inorganic sludge waste. A summary of the drums overpacked from March 11 through May 17, 1996 is given in Table I. The headspace gas analysis data for drums without pinholes in each content code showed that content code-3 had high amounts of volatile organic compounds (VOCs). Content code-1 drums also had amounts of selected VOCs significantly higher than other content codes but significantly lower than that in content code-3.
A large portion of the drums overpacked had package dates from 1980 through 1983. It was thought that the pinhole corrosion problem might be related to something that happened in this timeframe. The analysis on the database showed no correlation to the package date. Most overpacked pinhole corrosion drums had package dates from 1980 through 1983 because most of the accessible drums from content codes that exhibited this problem are from this same timeframe. Despite this, it is hypothesized that the corrosion would require a significant amount of time to penetrate the drum wall. The only database field to show a correlation to the overpacked pinhole corrosion drums, besides the high amounts of VOCs in content codes-3 and -1 headspace gas data, was 'matrix category.' This field simply contains a designation for the overpacked pinhole corrosion drums that represents absorbed organic liquids. This is understandable because the absorbed organic liquids are the VOCs.
VISUAL EXAMINATION
Visual examinations were used to characterize the observed failure mechanism. Photographs were taken as a record of some of these examinations (see Figures 1 and 2). Thirty one drums were visually examined. The visual characteristics of the pinhole corrosion observed were as follows: In a few cases, unbroken paint blisters were seen, which were approximately circular in shape with diameters of about 2 cm or smaller. These could be located in otherwise pristine areas of clean, painted drum surface or they could be next to many other broken paint blisters. In most cases, pinhole corrosion was observed as broken paint blisters with dried rust-colored streaks that emanated from pinholes about 2 mm in diameter or smaller. The metal surface under recently broken paint blisters exhibited a fresh surface with little to no surface corrosion, while that for older broken paint blisters exhibited severe surface corrosion. The combination of unbroken paint blisters on otherwise pristine areas of a drum and a near fresh metal surface appearance under recently broken blisters indicated that the corrosion originated internally, not externally.
The location of pinholes on the drums was overwhelmingly on the sides of the drum above the second roll ring corresponding to about the upper one-third of the drum (see Figure 1). Some drums had pinholes on the drum lid, fewer drums had pinholes on the mid-height region between the bottom two roll rings and very few drums had pinholes on the bottom third of the drum or on the drum bottom.

Fig. 1. Pinhole corrosion of a 55-gal CH-TRU waste drum.
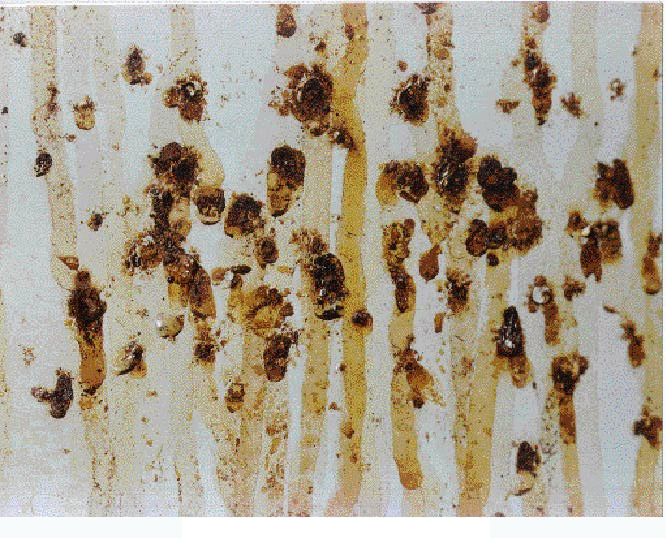
Fig. 2. Closeup picture of pinhole corrosion.
NONDESTRUCTIVE EXAMINATION (NDE)
The NDE techniques used in this investigation were neutron radioassay (RA), x-ray RTR and ultrasonic testing (UT).
Previous neutron RA measurements of a number of these CH-TRU waste drums showed they had little to no fissile material content, as expected.
X-ray RTR of a number of the IDC-003 drums showed they had type-II 90-mil rigid high-density polyethylene (HDPE) liners and a PE and/or polyvinyl chloride waste bag inside the liner. The HDPE liner has a 'milk bottle' shape with the shoulder of the liner starting just above the second roll ring of the drum (see Figure 3) as observed on the RTR display screen. Therefore, the region of the drum just above the second roll ring corresponds to the internal headspace of the drum between the rigid liner and the drum.
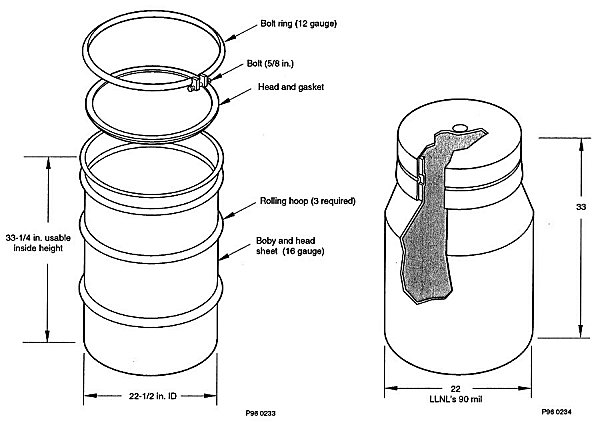
Fig. 3. Schematics of a) a 55-gal drum and b) a Type-II 55-gal drum liner.
The RTR operators are able to detect pinholes in the drum wall and liquids inside the drum. In a few cases of the content code-3 pinhole corrosion drums, liquids were observed inside the drum, but mostly between the waste bag and the rigid liner. In one case, a drum reported to have no pinholes by visual observation exhibited faint, small pinholes by RTR. When this drum was again visually examined, small paint blisters were readily observable on the drum but without associated rust-colored streaks .
Hand-held UT was performed on two pinhole corrosion drums (IDC-003). The one-half inch diameter UT probe was unable to distinguish any differences in drum wall thickness around or directly over pinholes.
CHEMICAL EXAMINATION
Chemical examinations were conducted on liquid effluent from the pinholes and on the drum headspace gas. The techniques used included: litmus paper, ion chromatography (IC), inductively coupled plasma - mass spectroscopy (ICP-MS), mass spectroscopy (MS), gas chromatography (GC), GCMS, titration, Fourier Transform Infrared (FTIR) spectroscopy and colormetric tube response.
Operations personnel made pH determinations by litmus paper on the rust-colored streaks and broken paint blisters while they were still wet. The pHs ranged from 0 to 3 as evidenced by the color change to the litmus paper. This indicated that the rust-colored fluid was highly acidic.
Liquid samples were collected from a few drums with pinhole corrosion and wet rusty streaks. One small sample (~5 microliters) was collected from three content code-3 (IDC-003) drums. Because there was so little sample, only anion analysis by IC was performed. The analysis showed a strong chloride presence with a trace (four orders of magnitude lower) of nitrate. A second liquid sample was also taken from a content code-3 drum. Cation analysis by ICP-MS showed it was high in iron and sodium with a little boron. Anion analysis by IC also showed a strong chloride presence.
Gas samples were collected from the headspace of a number of drums with and without pinhole corrosion. GC was used to determine the volume concentration of hydrogen, methane, alcohols and ketones. GCMS was used to determine the volume concentration of VOCs such as: carbon tetrachloride, chloroform, methylene chloride, trichloroethene, trichloroethane, toluene, trichloro-trifluoroethane (freon), etc. These analyses showed little hydrogen or methane in any of the drums, and that the content code-3 pinhole corrosion drums were high in VOCs and had a small amount of acetone. Additional analysis for the presence of hydrogen chloride (HCl) by silver chloride titration was inconclusive. It was suspected that any HCl would interact with the stainless steel of the gas sample canisters. When a gas sample from a content code-3 pinhole corrosion drum was collected in a plastic bag and analyzed by silver chloride titration, a positive HCl response was found.
Additional content code-3 pinhole corrosion drum headspace gas sample analyses with another MS system and by FTIR spectroscopy had limited success. The Gas Generation Testing System-MS analysis of a sample from a stainless steel canister showed a small HCl response. FTIR analyses of samples from both stainless steel canisters and plastic bags did not see HCl, but did see results similar to those by GCMS for the VOCs. The FTIR system was unable to detect HCl directly introduced into the system because it has stainless steel internals operating at 110ºC.
Headspace gas was also tested with colormetric tubes for HCl and moisture responses. Headspace gas was drawn through the drum filter and the colormetric tube via a teflon fitting with the aid of a calibrated pump. The tubes have a graduated scale in ppmv and contain a substance that changes color in response to HCl and to moisture, respectively. The previously described gas sampling and analyses collected gas samples with the aid of a needle through the filter into the headspace of the drum and therefore did not have to pass through the drum filter. A correction factor for barometric pressure via the ideal gas law was applied to the responses to calculate the volume concentration in ppmv. No correction factor for temperature was applied although a physical effect is suspected. Tests performed in a heated building showed higher responses, in general, than in unheated buildings. Positive HCl responses were mostly observed in content code-3 drums, and the highest proportion of these, as well as in the overall testing, were seen in drums with pinholes. The moisture colormetric tubes indicated the presence of moisture or water vapor in the few drums tested, at levels similar or slightly greater than that in the ambient air.
CALCULATIONS
Calculations for radiolytic dechlorination were performed using a conservatively assumed gas generation rate for chloride from the content code-3 waste. This resulted in calculated pHs similar to those measured by litmus paper for times ranging from one to six years. The actual gas generation rate is probably lower than the conservative assumption used. Therefore, the time range to reach the appropriate pHs would be longer also. This analysis is in agreement with the circumstances of the current problem because most accessible content code-3 drums have package dates from 1980 through 1983.
ANALYSES
A couple of analyses, for pitting corrosion as the source of pinhole formation and an overall corrosion scheme, were conducted to have a deeper understanding of the observed problem.
An analysis of pitting corrosion as the source of the pinhole formation was conducted. Pitting is localized corrosion of a metal surface, confined to a point or small area that takes the form of cavities. The pitting process causes changes in the chemistry inside the pit as compared to the bulk solution chemistry such that pitting corrosion rates can be orders of magnitude higher than the general corrosion rate. It was concluded that photographs of the leaking drums showed pitting corrosion that initiated on the drum interior. Also, the chemical analysis of the corrosion product identified aggressive anions and acidic pH which is characteristic of active pitting corrosion.
A literature review analysis on possible corrosion mechanisms was also conducted. The topics covered in the literature review were: adsorption phenomena on iron surfaces, corrosion mechanisms, electrochemical reactions, and halocarbon interactions with reducing agents.
A four-phase process was hypothesized for the observed pinhole corrosion. In the first phase, the inner drum surface is partially corroded from fabrication. The surface corrosion consists of a mixture of iron oxides and hydrated iron oxides with intermittent surface discontinuities. In the second phase, water, oxygen, halocarbons and HCl acid attack the exposed iron and oxo-iron surfaces at discontinuities and other areas such as stress points. This attack results in localized or pitting corrosion, the formation of soluble corrosion byproducts and corrosion products that can participate in further corrosive reactions. The third phase involves several reactions directly causing corrosion or generating corrosive byproducts. The number of reactions and the vigor in which they proceed increase dramatically. The fourth phase represents pit penetration into the drum wall and continues until it penetrates the entire drum wall thickness and causes the exterior paint to blister.
The reactions suspected as initially operable in the CH-TRU waste drums (second phase) were: radiolysis, direct reduction of halocarbons, and reductive dehalogenation. A number of other possible reactions were investigated but deemed not as likely as the above three reactions. All reactions investigated are well documented, but their rates and competitiveness with other reactions are impossible to predict with any certainty.
CONCLUSIONS
CORRECTIVE ACTIONS
The corrective action taken upon discovery of pinhole corrosion drums was to overpack these 55-gal drums into 83-gal drums by the end of the day. Because HCl was forming in the drum headspace, a simple test was devised to check for its presence. Colormetric tubes containing a substance that reacts with HCl and changes color were fitted to the drum filters. Headspace gas was drawn through the drum filter into the colormetric tube via a hand pump. Any change in color of the substance in these colormetric tubes indicated the presence of HCl. Hundreds of drums of various content codes were tested in this manner. It was concluded that only content code-3 drums had a significant probabilty for the presence of HCl in their headspace gas. These drums were segregated in storage to allow ready inspection and efficient handling, if needed.
LESSON LEARNED
It is recommended that any facility involved in the long-term storage of waste or other contents, that include chlorinated VOCs in unlined steel containers, be wary for the possible development of pinhole corrosion.
REFERENCES
ACKNOWLEDGEMENTS
This work was funded under the U.S. Department of Energy Idaho Operations Office Contract number DE-AC07-94ID13223.