THE CERBERUS PROJECT:
STUDY OF THE EFFECT OF HEAT AND RADIATION ON THE
NEAR FIELD OF A HLW OR SPENT FUEL REPOSITORY
L. Noynaert, P. De Cannière, G. Volckaert and D. De Bruyn
SCK•CEN
Boeretang 200
B-2400 Mol, Belgium
C. Beaucaire and H. Pitsch
CEA, Centre d'Etudes de Saclay
LIRE, Bât. 450, F-91191 Gif-Sur-Yvette
Cedex, France
A. Bouchet and J.C. Parneix, ERM
avenue du Recteur Pineau 40, F-86022 Poitiers
Cedex, France
ABSTRACT
The Cerberus test was the first combined demonstration test performed from an underground laboratory installed in a plastic clay formation. The near field effects appear to be limited on the clay itself. Irradiated and heated Boom Clay appears to have a similar thermo-hydro-mechanical behaviour as Boom Clay heated at the same temperature. However, pyrite oxidation products found in the interstitial clay water, as thiosulfate, may play a detrimental role for the lifetime of the metallic barriers (pitting corrosion). The first results indicate an increase of the corrosion-rate of carbon steel.
INTRODUCTION
The CERBERUS project (Control Experiment with Radiation of the BElgian Repository for Underground Storage) was developed by the SCK•CEN under contract with the European Communities (EC) and with the Belgian Waste Management Authority (ONDRAF/NIRAS).
The Cerberus test is aimed at simulating the near field effects in an argillaceous environment of a Cogema HLW canister after 50 years cooling time. The gamma activity of this canister mainly arises from 137Cs and its daughter 137Ba (1.53 PBq per canister for each isotope) and produces still a thermal power of 580 W. These properties were simulated during 5 years (1989-1994) in the Cerberus mock-up using a 60Co-source of 400 TBq and 2 electrical heaters dissipating each 363 W.
The test was designed to collect experimental in situ data about
thermo-hydro-mechanical couplings in an argillaceous formation under thermal loading. In
situ experiments were also performed to determine the thermal and hydraulic transfer
parameters, to measure the
pH/Eh-values, and to detect the presence of gases produced by radiolysis as H2
and CH4. A migration test using a cocktail of 241Am and 99Tc
was also performed in the near field of the Cerberus test. At the end of the hot
phase of the Cerberus test, the behaviour of engineered barriers, i.e.
backfill material, waste forms, overpack and canister materials as well as the behaviour
of the Boom Clay were studied on a basis of samples.
The Cerberus test was the first combined demonstration test performed from an underground laboratory installed in a plastic clay formation. The near field effects appear to be limited to the clay itself. However, pyrite oxidation products found in the interstitial clay water, as thiosulfate, may play a detrimental role in the lifetime of the metallic barriers (pitting corrosion). The analyses of the metallic rods are still in progress. Results will be available in the near future and will precise more quantitatively the consequence of the pyrite oxidation.
PRESENTATION OF THE CERBERUS TEST
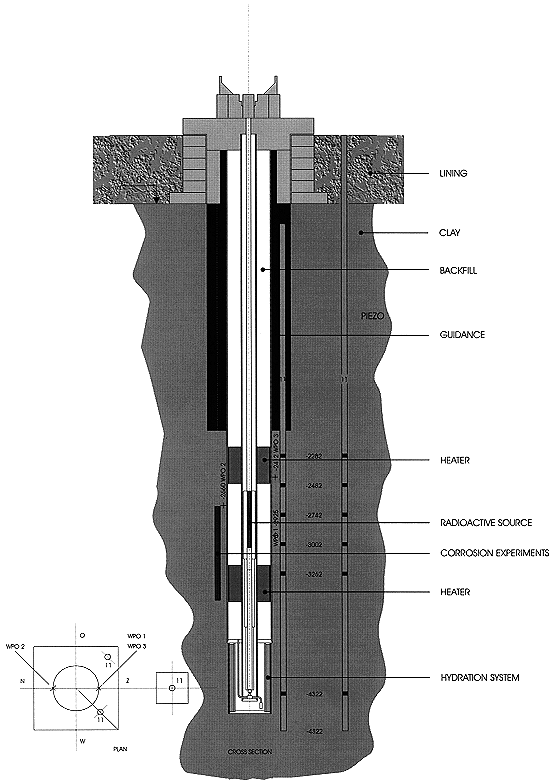
The detailed lay out of the test device shows the following characteristics (Fig. 1, [1], [2]):
Fig. 1. Cerberus Test.
RESULTS AND DISCUSSION
The Cerberus test [2] was started more than 1 year after the drilling of the boreholes and the installation of the mock up device and its instrumentation. The experimental set up was loaded by the six 60Co-sources (mean activity 67 TBq) in almost 2 weeks. Then after 1 month, a constant power supply of 363 W was delivered at each electrical heater during 5 years.
Radiation field
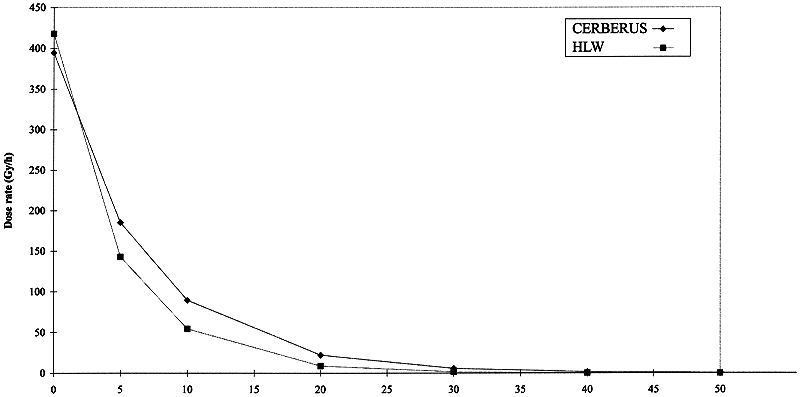
Figure 2 compares the computed dose rate in the clay, located at the horizontal mid plane of the Cerberus mock up, with those predicted for a HLW canister. The simulations are satisfying in the zone where most of the measuring probes are installed. The clay around the Cerberus test was submitted during 5 years at dose rates up to 400 Gy/h (in contact with the canister wall). The total absorbed dose at this location reaches 17 MGy. The dose rates were measured by means of an ionisation chamber. They were also predicted using the program DOSEGEO. The predicted dose rates were in good agreement with the values measured during the period of the test.
Thermo-hydro-mechanical field
The maximum temperatures reached during the test were:
As shown on Fig. 3, the computed temperatures using the program TEMPPRES are generally in good agreement with the measured values. The mean error is only 5 % of the measured values.
The pore water pressure response to the heat transfer depends on the location and, in particular, on the distance to the heating elements. Two different behaviours were observed:
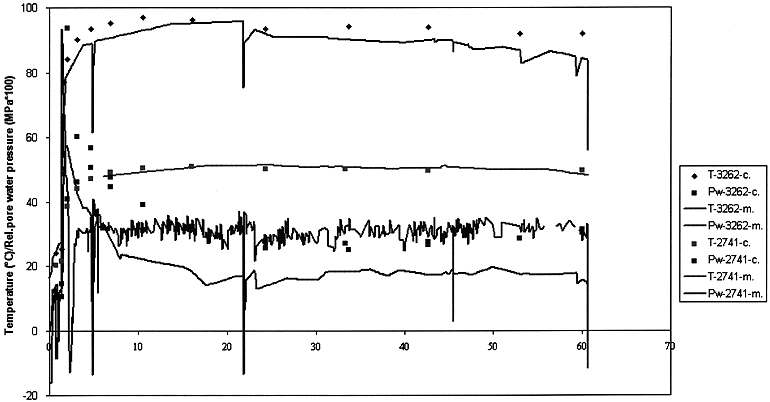
Fig. 3. Comparison between calculated (-c-) and measured (-m-) temperature and pore water pressure.
The pore water pressure evolution at the screen PSW3262 was computed using the program TEMPPRES with the best data available in 1992 (Fig. 3.). The maximum pore water pressure due to the thermal loading can be well reproduced. Its fast dissipation, which cannot be simulated is due to the creation of micro-fractures. Indeed, the hydraulic conductivity measured after 1 year thermal loading has increased by almost 50 % above what can be expected by the temperature increase. Then, a slight decrease of the hydraulic conductivity was observed as a function of time. At the end of the thermal loading, the hydraulic conductivity was very close to the value measured before the start of the test. This indicates that Boom Clay remains plastic and has a self healing capacity after fissuring.
At the screen PSS2742, the general trends can be simulated by the program TEMPPRES, the maximum value can be reproduced in amplitude but not in time and the pore water dissipation occurs faster than the predictions. The hydraulic conductivity measured after 1 year thermal loading has not increased as much as expected by the temperature increase. Then a slight decrease of the hydraulic conductivity was observed as a function of time. At the end of the thermal loading, the hydraulic conductivity was 20 % below the value observed before the start of the test.
By comparing the temperatures and the pore water pressures, measured at the points PSW3262 and PSS2742, one can conclude that the pore water pressure at any place is more sensitive to heat transfer variation than the temperature, i.e. pore water pressure can be modified by a change of heat transfer at places where temperatures remain unchanged.
The shutdown of the electrical heaters and the retrieval of the 60Co-sources induced almost the same pore water pressure drops as observed during the main power supply failures, but in this case the restoration of the pore water pressure pattern is governed by the local conditions around the Test Drift. After 15 months cooling time, the pore water pressures have not yet reached a quasi-steady state. They remain at values 35 % lower than the level reached at the end of the hot phase of the Cerberus test.
The influence of a temperature increase on the mechanical properties of Boom Clay at depths that represent a HLW disposal is currently being studied and results have been already published [13]. Heating a clay sample from 20 to 80 °C leads to a marked decrease (up to 28 %) in its mechanical strength. The extent of the Thermally Disturbed Zone (TDZ) surrounding a HLW disposal gallery would then increase, what should be taken into account in the long-term performance calculations.
Samples of Boom Clay have been taken from the URL at Mol, in the vicinity of the Cerberus experiment and tested in the laboratory to investigate the influence of an in situ heating and/or radiation field on the mechanical properties. Reference samples (undisturbed by heating or radiation) have been cored from the URL at the same depth.
The Mohr's circles related to the reference samples (Fig. 4, black colour) indicate a shear strength c of 0.9 MPa and an angle of shearing strength F of 4°. These tests are in very good agreement with similar tests performed in the past on the same material. Samples submitted to heating (Fig. 4, red colour) or radiation (Fig. 4, blue colour) show systematically a lower resistance. Assuming F remains unchanged, the residual shear strength should amount to 0.6 - 0.7 MPa (heated samples) or 0.6 MPa (irradiated sample).
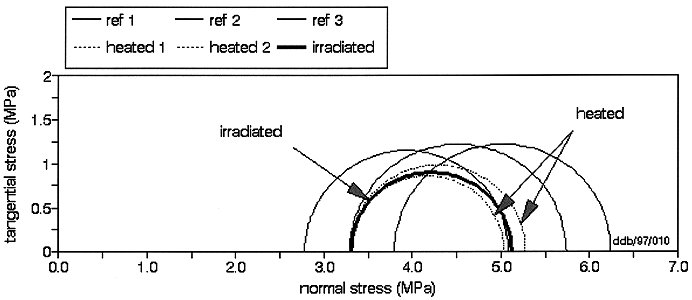
Fig. 4. MOHR's Circles of the samples tested in the framework of CERBERUS 3: Comparison between undisturbed, heated and irradiated samples.
Chemical Field
Effects of heat on interstitial fluids
Interstitial fluids were sampled in the near field of the Atlas experiment to evaluate the chemical evolution of interstitial water with temperature. Samples were taken at the Atlas piezometer screens #85 and #93, at 15.85 m depth (from the gallery extrados). The measured temperature is 35 °C. Different samplings were carried out. Except the first sampling, which is likely not significant, the chemical composition remains stable and is very similar to the reference composition of interstitial fluid issued from piezometer Archimede 1 [3]. The concentrations of the main components of the waters are summarised in Table I. We observe that silica is the most sensitive element. From 17 °C to 35 °C, the silica concentrations increase, keeping the solutions close to the saturation of the chalcedony over this range of temperature. To a lower extent, Na and K increase also leading to a lower Na/K ratio.
Table I. Comparison between interstitial water around ATLAS experiment and from ARCHIMEDE piezometer. The concentrations are expressed in mM.
Experiment |
Atlas 1 |
Atlas 2 |
Archimede 1 |
in situ T (°C) |
35 |
35 |
17.2 |
pH (measured in laboratory at 25°C) |
8.25 |
8.32 |
- |
pH (measured in situ) |
- |
- |
8.2 |
[mM] |
[mM] |
[mM] |
|
HCO3- |
14.5 |
13.9 |
12.1 |
Cl- |
0.47 |
0.46 |
0.50 |
F- |
0.16 |
0.16 |
0.12 |
SO42- |
0.09 |
0.01 |
0.03 |
Br- |
0.004 |
0.004 |
0.007 |
Si(OH)4 |
0.38 |
0.38 |
0.13 |
Na+ |
13.6 |
13.9 |
12.4 |
K+ |
0.36 |
0.37 |
0.22 |
Mg2+ |
0.10 |
0.07 |
0.05 |
Ca2+ |
0.09 |
0.05 |
0.07 |
Li+ |
0.075 |
0.075 |
0.004 |
Effects of heat and irradiation on interstitial fluids
The Boom Clay around the Cerberus experiment can be considered as slightly oxidised by the water gamma radiolysis or/and the intrusion of oxygen. The evolution of the main chemical characteristics during the overall experiment has already been described in detail [2], and is summarised in Table II. After a first stage of pyrite oxidation leading to the release of sulphate in solution and acidification, the fluids evolve again towards Na-HCO3 type, with a neutral pH and still reducing Eh. Calculations of chemical speciation and mineral phases equilibria show that the solutions are saturated at high temperature as at low temperature, with respect to the same mineral assemblage, which constitutes the buffer capacity of the Boom Clay. The calculated higher pCO2 in the last stage of the Cerberus experiment is likely due to the dissolution of carbonates in the first step of acidification. It appears that this CO2-excess is not immediately resorbed in the clayey matrix, keeping the final solution close to the neutrality.
Table II. Evolution of the Main Chemical Characteristics of Interstitial
fluids
During an Oxidizing Pperturbation.
| Framework of the study | Initial conditions at low temperature ARCHIMEDE [3] |
Strong oxidizing perturbation
|
Effects of heat and radiations at high temperature (80°C) CERBERUS II [2] |
| Main chemical characteristics of interstitial fluids | Na-HCO3 pCO2: 10-2.4 atm pH: 8.2 Eh: -240 mV (SHE) |
Na-SO4 pH ~ neutral/acid |
Na-HCO3 pCO2: 10-0.5 atm pH: 7.2 Eh: -280 mV (SHE) |
| Main mineral phases at equilibrium | chalcedony (Si) calcite (Ca) dolomite (Mg) goethite (Fe III) siderite (Fe II) pyrite (S) |
pyrite oxidation carbonate dissolution ion-exchange (clay minerals) |
chalcedony (Si) calcite (Ca) dolomite (Mg) goethite (Fe III) siderite (Fe II) pyrite (S) |
Due to radiolysis and/or corrosion, hydrogen has been produced in the near field of the test. Concentrations from 0.15 to 7.55 µg/kg water were measured. No significant increase of methane was detected.
A migration experiment using 241Am which is representative of the radionuclides strongly complexed by humic acids and 99Tc which is representative of redox sensitive nuclides was performed in the near field of the Cerberus test to assess the effect of heat and radiation on the retention properties of Boom Clay. Comparison with a similar experiment performed under natural in situ conditions has not shown any significant differences.
To assess the effect of heat and radiation on waste forms (glass and concrete), metallic canister (CS, SS) and overpack materials (Ti, Ti/Pd, Cu, Hastelloy, ...), specimens were submitted during 5 years to heat and radiation. Their analyses are still in progress. An increase of the corrosion rate of carbon steel is already observed.
Influence of heat and radiation on mineralogy
The clay analysis is performed on four samples by means of X-ray diffractometry (XRD) associated with the following techniques: chemical analysis of major elements by atomic absorption spectrophotometry, measurement of the cation exchange capacity (CEC), and determination of the carbonate content by calcimetry. A petrographical study is done in optical and electronic microscopy on thin sections made on clayey materials previously hardened. To take into account the possible effect of the heat and radiation gradient, clay material was sampled at different distances (0-2 mm; 2-5 mm; 19-21 mm and 35-40 mm) from the canister wall.
The global analysis of the samples by the above mentioned techniques does not indicate any strong mineralogical evolution among the major components of the sediment: phyllosilicate (illite-smectite random mixed layer, illite, kaolinite and micas), quartz and calcite to a lesser degree. XRD analyses on bulk samples do not indicate any variation on the main mineralogical components. The presence of gypsum only in sample 2-1 is due to a local concentration of large crystals of sedimentary origin, as attested by the observation of the thin section under the optical microscope.
The comparison of XRD data on oriented glycolated samples taken at different distances from the heat and radiation source show that the intensity of the peak corresponding to the swelling phase tends to decrease near the sources. This phenomenon could be due to either an increasing percentage of 10 Å layers or a decreasing size of crystals. All this information should be checked with more precise mineralogical and petrographic investigations.
In the literature, mineral transformations induced by heating, and affecting clay sediments constituted from illite/smectite, correspond more often to illitisation reactions [4, 5] of the type "smectite -> I/S -> illite", or "I/S -> illite" according to the degree of progress of diagenesis of the material. A transformation affecting disordered mixed layer illite/smectite as those present in the Boom Clay will first lead to the formation of ordered mixed layer illite/smectite (~ 50% of smectite maximum). This transformation has been described in diagenesis for temperatures of at least 90-100°C [6, 7, 8, 9], in hydrothermal alteration, at around 110°C [12], and in contact metamorphism near 150°C [10]. Under favourable chemical conditions, experiments at high temperature give results similar to those observed in nature. However, to reproduce experimentally, but in a shorter time, the same transformations as in natural phenomena, the temperature must be much higher to obtain ordered mixed layer I/S [11].
The results of experiments (corrosion loops) made in Mol during 4 and 7 years at ~ 90 °C are in agreement with the literature data because no transformation of mixed layer illite/smectite (R = 0 initially) can be detected. Except possible facies changes, data are similar to these previously obtained (by deconvolution of X-rays diffractograms) on undisturbed fresh samples of reference.
However, alteration processes around sulphur (frambohedral pyrite) could be observed and may be due to thermal effects. The millimetric framboids of pyrite are separated from the clay matrix to be examined individually in optical microscopy: they generally present a homogeneous aspect. Gypsum crystals are present in contact with some framboids. They form agglomerates whose size may be of the same order of magnitude than that of the surrounding framboids (i.e., ~ 0.1 mm). These sulphates are too less abundant to be detected by X-rays diffraction on powder.
Mineralogical reactions have been put in evidence around some large pyrite framboids: a brown halo enriched in iron has developed in the clay matrix, to whom corresponds also a zone depleted in calcite. The presence of fissures in the neighbourhood of some framboids has been observed: however, we ignore if these fissures already existed in situ in the original sample during the thermal test, but even if their size has further increased, they might constitute physical discontinuities in the rock making the circulation of fluids possible.
Complementary studies are needed to give more precision on the origin of these transformations. The micro-petrographical approach is certainly the more appropriated to detect the small transformations occurring in the clay and to put in evidence the existence of preferential micro-paths for fluids.
CONCLUSIONS
The clay around the Cerberus test was submitted during 5 years to dose rates up to 400 Gy/h (in contact with the canister wall). The total absorbed dose at this location reaches 17 MGy. During this period, temperatures up to 120°C were reached. The dose-rate measured can be well simulated by the program DOSEGEO. The temperatures have been modelled by the programme TEMPPRES assuming a constant value for both the thermal conductivity and diffusivity. Pore water pressures are more sensitive to the dissipated heat than the temperatures themselves. The hydraulic perturbation due to heat transfer variation propagates faster than the temperature front. Micro cracks can be induced in the clay: it occurs where the pore water pressure increase induced by the heat reaches the local effective stress. Nevertheless, Boom Clay has shown self healing properties. After five years of continuous irradiation and heating the physico-chemical conditions of the Cerberus test have not yet evolved to strongly oxidising conditions: the pH remains about neutral while the redox potential is still reducing. A slight oxidation of Boom clay occurs around Cerberus, as indicated by the slight acidification, the less negative Eh-value and the chemical conditions measured in the Cerberus water. The interstitial clay water changes progressively from a sodium bicarbonate type to a sodium sulphate type, due to the pyrite oxidation induced by the radiation and the heating fields. Species relevant for some problems concerning the near field integrity have been detected; sulphur species and thiosulphate for the metallic barrier corrosion, oxalate or small organic anions for the radionuclides complexation (faster migration), boron or silicon chemistry for the glass matrix corrosion (dissolution, diffusion and sorption of borate or silicate in Boom clay).
REFERENCES