SIMPLIFIED MELTER SYSTEM PROPOSED FOR
VITRIFICATION OF INEEL HIGH-LEVEL WASTE
G. E. Stegen
Cogema Technologies, Inc.
Idaho Falls, Idaho
S. L. Lambert
SGN Eurisys Services Corporation
Richland, Washington
S. Goetghebeur, Ph. Kalousdian
SGN Reseau Eurisys
Saint Quentin en Yvelines, France
ABSTRACT
The Idaho Engineering and Environmental Laboratory (INEEL) is evaluating options for the processing and disposal of stored high-level waste calcine and high-activity liquid tank wastes. An engineering study on vitrification of high-level wastes was developed as part of a feasibility study on INEEL waste treatment performed in 1997. An improved vitrification process and facility concept was developed that is expected to reduce capital and operating costs, reduce waste generation, and improve overall operating efficiency compared to previous designs. Key to these improvements is the cold crucible melter (CCM). This advanced melter has been developed and proven through thousands of hours of testing, and is now entering the industrial implementation phase. Recent testing and engineering studies have shown that for many applications, a simpler, lower cost option is to directly feed concentrated liquid or slurry waste to the CCM rather than use a separate calciner to precondition the melter feed. This paper describes the proposed vitrification concepts, and discusses considerations that led to selection of the recommended vitrification system.
INTRODUCTION
Spent nuclear fuel (SNF) was reprocessed at the Idaho National Engineering and Environmental Laboratory (INEEL) from 1953 through the 1980s. A substantial quantity of residual wastes from reprocessing has accumulated and remains to be treated and disposed. Most high-level waste (HLW) raffinates from reprocessing have been solidified and are stored as granular calcine solids in stainless steel bins. Calcine inventory is estimated at 4,836 cubic meters or about 7,000 metric tons after the small amount of remaining HLW raffinate is calcined in 1998.
High-activity liquid Spent nuclear fuel (SNF) was reprocessed at the Idaho National Engineering and Environmental Laboratory transuranic (TRU) waste is also stored in tanks at INEEL. This waste has a relatively high sodium content, and is referred to as sodium bearing waste (SBW). The sodium content makes it difficult to process in the existing HLW calciner system. An estimated 3,000 cubic meters of SBW will remain after planned HLW calcination, waste consolidation, and evaporation campaigns are completed. An additional 1,300 cubic meters of concentrated liquid TRU waste is expected to be generated from future decontamination and site operations through 2012.
A major feasibility study on INEEL waste treatment was performed in 1997 for the U. S. Department of Energy (DOE). Lockheed Martin Idaho Technologies Company (LMITCO) was responsible for overall management of the study, which was performed by Fluor Daniel with support from an SGN team which included SGN Reseau Eurisys (St. Quentin Yvelines, France) and its affiliates, SGN Eurisys Services Corporation (Richland, Washington), and Cogema Technologies (Idaho Falls, Idaho). The SGN scope included development of HLW vitrification process and facility concepts, which are summarized herein and described in more detail in the study report1.
The study evaluated an option that uses efficient separations processes to divide SBW and calcine into two product streams: a relatively small volume high-activity waste (HAW) stream to be vitrified and disposed in a geologic repository; and a relatively large volume low-activity waste (LAW) stream to be immobilized in grout for near surface disposal. The separations and LAW immobilization plants would start operation with SBW before startup of the calcine retrieval, calcine dissolution, and HAW vitrification plants. Early processing of SBW is intended to accomplish removal of liquid tank waste from the existing tank farm by 2012 to comply with an agreement between the State of Idaho, the DOE, and the U. S. Navy. This option, referred to as "early separations," is one of several options being considered by the DOE in an Environmental Impact Statement currently being prepared.
VITRIFICATION FEEDS
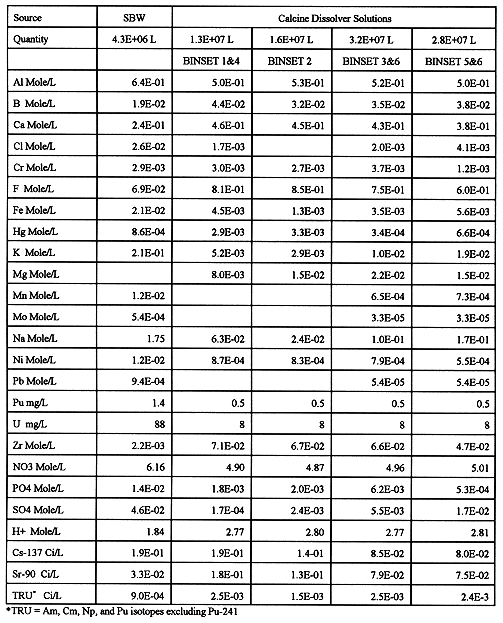
Feeds to the HAW vitrification plant come from the separations process, and feed characteristics are significantly affected by separations technology and process flowsheet. Separations technology currently being tested at INEEL was defined by LMITCO as the basis for the study2,3,4. The separations processes can directly process SBW, however, calcine must be dissolved in nitric acid prior to separations. Compositions estimated for calcine dissolver solutions and liquid SBW feed to separations are shown in Table I. Separations processes and resulting HAW feeds are discussed below.
Table I. Average Liquid Waste Feeds to Separations

Solids Removal
Cesium Removal
Solvent Extraction
Depending on feed composition and process conditions, TRUEX solvent may also extract a significant fraction of some non-radioactive chemicals, including Hg, Fe, Zr, Ru, Pd, Y, lanthanides, and nitric acid. In the flowsheet used as the study basis, the solvent is stripped with a 0.01 M solution of 1-hydroxyethane 1,1-diphosphonic acid (HEDPA) in dilute nitric acid. The HEDPA strip removes most extracted components. An exception is Hg, most of which is removed by a dilute sodium carbonate scrub solution. The spent sodium carbonate solution is processed to recover Hg as a separate small volume waste. The SREX solvent also extracts significant portions of other components, including Ba, K, Pb, Hg, Zr, and nitric acid. The SREX solvent is stripped with dilute nitric acid and/or ammonium citrate solutions. Solvent strip solutions from TRUEX and SREX constitute the main feed for HAW vitrification. Optimization of separations processes to minimize components in these streams that are undesireable for vitrification is therefore a primary objective of separations process development work9.
Solvent strip solutions from SREX and TRUEX are concentrated by evaporation, blended with cesium ion exchange eluent, and transferred to storage tanks prior to transfer to HAW vitrification. Estimated composition of this combined liquid HAW stream is shown in Table II for SBW and a typical calcine waste.
Several aspects of waste compositions in Tables I and II are worth noting. Calcine dissolver solutions contain relatively high levels of zirconium, aluminum, calcium, and fluoride. Zirconium and aluminum result from typical INEEL reprocessing flowsheets in which fuel and cladding are dissolved together so that cladding materials report to reprocessing raffinates along with the fission products. Fluoride was added during reprocessing and calcium was added during calcination to control fluoride volatility and corrosion. Mercury added during reprocessing is present at significant levels in SBW and some calcines.
Table II. Waste Feeds to HAW Vitrification
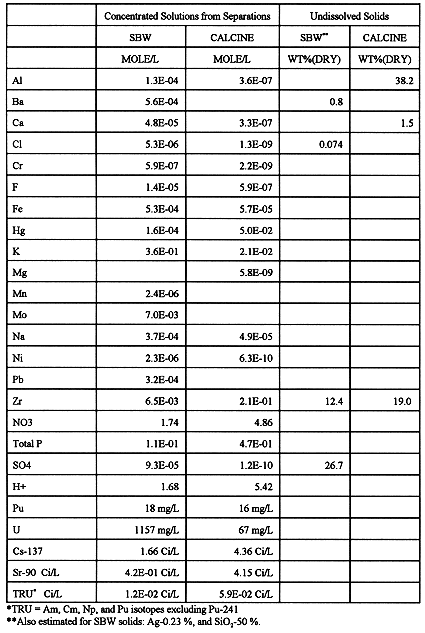
The sulfate concentration shown for SBW solids is very high and could pose a problem for vitrification because of low sulfate solubility in borosilicate glass. For the study, it was assumed that SBW solids will be blended with calcine solids in order to avoid unacceptably high sulfate in glass. However, the need for this is uncertain. The high sulfate concentration shown is an estimate based on thermodynamic calculations. Actual analyses for sulfur in undissolved solids are not yet available for either SBW or calcine.
From the perspective of vitrification, the high phosphorus concentration from HEDPA dominates the strip solution compositions for both SBW and calcine. Depending on the actual separations process performance, zirconium content of calcine and potassium content of SBW strip solutions may also be important. Recent work to optimize the separations processes has focused on reduction of phosphorus and zirconium in the TRUEX strip solutions9.
MELTER TECHNOLOGY SELECTION
Selecting a melter technology requires consideration of factors such as expected feed composition, desired glass properties and composition, required processing capacity, nuclear facility operation and maintenance requirements, and cost of waste disposal. The study scope and schedule were limited, so that formal trade-off studies could not be performed on design alternatives. Recommended melter technology and other process and facility concepts were therefore based on the judgment of vitrification experts from SGN and affiliated companies with extensive experience in evaluation, development, design, and operation of nuclear waste vitrification systems.
The quantity and composition of vitrification plant feeds were provided by Fluor Daniel, and LMITCO provided information on the approximate glass composition range and desired waste loading in glass. The desired products are relatively high phosphorus borosilicate glasses, with P2O5 content in about the 10 Wt % range. These glasses may form two glassy phases on cooling, but usually have been found to meet HLW repository durability requirements5 . Using forecast values for separations performance and waste loading in glass, overall production is relatively small, about 1.3 million kg of HLW glass over the expected 20-year operating life of the plant.
A cold crucible induction melter was recommended for this application by SGN based primarily on the need to process potentially corrosive glasses with high phosphate and sulfate, the ability to easily handle required processing rates with a single melter, and the significant advantages of the CCM for remote nuclear applications (discussed in more detail below). A slurry-fed melter was recommended for the INEEL application because the required processing rate is easily met with a single melter with no precalcining of melter feed. A slurry-fed meter system is simpler and less expensive than a system with both a calciner and melter.
FEATURES OF THE COLD CRUCIBLE MELTER
A key element of the vitrification process recommended by SGN is the CCM. Development work on the CCM for nuclear waste vitrification was initiated in France6,7 and Russia8 to improve on available melter technologies for nuclear waste vitrification. Features of the CCM are discussed below.
For the INEEL study, a 1-meter diameter slurry fed CCM is recommended. Nominal capacity of a 1-meter CCM is approximately 100 kg glass per hour with dry feed. For slurry feeding this capacity is conservatively derated to about 30 kg glass per hour, or a maximum of 60 L/hr of liquid feed.. Higher glass production can be achieved by concentrating the feed to a high solids content, for example by using a wiped film evaporator8. However, the additional complexity is not needed for this application. Using the forecast glass production of about 13,000 metric tons over 20 years, the INEEL application requires an average glass production rate of approximately 7.4 kg per hour before allowance for down time. The recommended melter can easily handle the required production rate and, in fact, provides surplus capacity as a contingency
OVERALL PROCESS DESCRIPTION
Liquid feed is batch transferred into a buffer tank from the separations plant via a pipeline. Two slurry streams are also delivered by pipeline: the SBW solids; and a mixture of spent ion exchange media and undissolved solids from calcine. The slurry feeds are kept segregated to allow each to be blended into the melter feed at the desired ratio.
Liquid feed is transferred to a melter feed tank where it is concentrated by evaporation, chemicals are added if needed, and it is mixed and sampled. The liquid feed, the two slurry feeds, and recycle liquor from the off-gas dust scrubber are metered to a confluent pot where they mix and drain by gravity into the melter. Dry glass-forming frit is added separately to the melter in small metered batches every several minutes.
The CCM is equipped with a mechanical stirrer to assure good mixing of waste and frit, and to improve the melting process. A nominal 1250 C melting temperature is maintained and glass is periodically batch poured into a canister through a bottom drain. Steam and decomposition gasses flow out through the top of the melter to a dust scrubber where they are scrubbed with hot recirculated acidic scrub liquor. A small stream of the scrub liquor is continuously purged and recycled to the melter feed. From the dust scrubber, the off-gas flows through a cooler, two scrubbing columns, a demister and HEPA filters. Residual nitrogen oxides in the filtered offgas are destroyed by reaction with ammonia in a selective catalytic reduction unit, and the treated off-gas is discharged to the stack.
Canisters filled with glass are cooled and then sealed with a cap that is welded in place. Canister external surfaces are decontaminated using a water wash followed by an abrasive slurry that removes the oxide surface layer, and then another a water rinse. After a smear test to verify external surfaces have been decontaminated, the canisters are finished and ready for loadout or storage.
FAILED EQUIPMENT HANDLING
The study design provided the capability for dismantling, size reduction, decontamination, sorting, and packaging failed melters and other melter cell equipment. This is typical of SGN-designed HLW plants, and is found to be an efficient and cost effective waste management practice. Including this capability in the initial plant design reduces the need for storage and double-handling of failed equipment, and maximizes the fraction of the solid waste that can be directly disposed as low-level waste. Use of the slurry-fed CCM allows the melter and equipment dismantling functions to be provided with relatively small remote cell space. In the preliminary concept developed for the study, the melter, off-gas dust scrubber, all equipment required for dismantling and decontamination of failed equipment from the melter cell, and equipment for activity measurement to allow segregation by activity level were located within a single 4-meter by 18-meter cell. Required remote cell space and remote handling equipment are significantly less than would be required to provide similar functions for a ceramic melter of similar capacity.
REFERENCES