VOLATILIZATION OF ALPHA ACTIVITY DURING
HEATING OF RADIOACTIVE ASHES
A.S. Aloy, V.Z. Belov, B.S. Kuznetsov
KRI
St Petersburg, Russia
D. Gombert, D. Knecht
LMITCo
Idaho Falls, USA
Yevgeny Macheret
Department of Energy
Idaho Operations Office
ABSTRACT
Thermal treatment of mixed wastes is one option that has the potential to significantly reduce final disposal volume and increase the durability of the waste form in one operation. One potential concern in evaluation and design of such a system, however, is the volatilization of contaminants of concern, which may increase secondary waste generation, cause permitting concerns, and complicate design of engineering containment. To evaluate the potential for significant contaminant losses, ash from mixed waste incineration was stabilized by melting at 1300oC while offgases and evolved particulate were captured for analysis. Losses of gamma emitting radionuclides were up to 20% and dominated by cesium, but losses of plutonium and uranium were less then 0.1%.
INTRODUCTION
Incineration of combustible radioactive waste reduces volume significantly, yielding ash residues containing up to 99 % of the radionuclides. Both bottom and fly ash are unsuitable for long-term storage or final disposal because of their physical form (easily spread and ingested finely divided solids), low chemical stability (both TCLP and ANSI 16.1), and low compressive strength (ASTM C-39).
Several options are available to enhance the physiochemical properties of ash residues resulting in a solid matrix that readily passes compressive strength requirements, and can be made to pass leach criteria for RCRA toxic and radioactive constituents. At various international sites this is achieved by bituminization [1] or by grouting [2]. Some methods are also being developed for the fixation of ash residues in stable ceramic matrices based on natural clays [3-6]. However, these processes generally increase the final disposal volume, which typically causes an increase in overall cost.
One promising option leading to a significant volume reduction of ash residues is melting using only sufficient additives to result in an acceptable waste form. One such technology to immobilize ash residues by partial melting in the presence of fluxing additives uses induction melting in a water-cooled crucible. This process is in practice on an industrial scale at the SIA "Radon" (Moscow) [7,8].
One design issue to be evaluated prior to implementation of any high-temperature process for radioactive waste treatment is transfer of radionuclides in the vapor phase due to aerosol carry-over and volatility of some compounds. The distribution of radionuclides between the final product and the gaseous phase is a decisive parameter in designing the offgas system, evaluating the quantity of secondary waste, and determining a safe range of operating conditions.
This paper covers the results of experimental studies funded by the Mixed Waste Focus Area to quantify the distribution of radioactive isotopes between the gaseous phase and the final product in the heat treatment of real ash residues.
EXPERIMENTAL EQUIPMENT
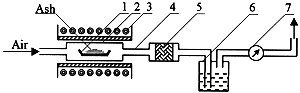
The design of the experimental system is shown in Figure 1. A weighted ash sample was placed in a ceramic cuvette (1) inside a quartz ampoule (2) placed in an electrically heated tube-furnace (3). Air was used as the sweep gas, passing through the furnace and out via quartz tubing (4) a filter packed with superfine glass wool (5) and a bubbler containing 1 M HNO3 (6). Airflow was controlled by a measuring device (7). After passing through a catching system air was pumped into a system of exhaust ventilation. Temperature in the resistance furnace was controlled by Pt / Pt-Rh thermocouples.

Fig. 1. The scheme of laboratory unit for volatilization study
1 - Ceramic Cuvette; 2 - Quartz Ampule; 3 - Resistance Furnace; 4 - Quartz Tubing; 5 - Filter; 6 - Bubbler; 7 - Airflow Meter.
RESULTS AND DISCUSSION
Isotopic Composition of Ash
The isotopic composition of the radionuclides in the ash from a commercially operated controlled-air, fixed-hearth incinerator burning miscellaneous combustibles from industry, hospitals and research institutes which use radioactive materials was determined by gamma spectrometry, alpha spectrometry, and by measuring the total alpha activity with a luminescent substance (ZnS(Ag)). Ash samples of 1-2 grams were placed in a special container for gamma spectrometry and measured in the well of a high-purity germanium detector. The radionuclide content in the starting ash measured by gamma spectrometry is shown in Table I.
Table I. Radionuclide Concentrations in Ash by Gamma Spectrometry.
| Isotope | 137Cs | 134Cs | 113Sn | 144Ce | 54Mn | 60Co | 152Eu | 238U | 235U | 239Pu | S a |
| Bq/g | 360 ± 29 |
18 ± 3 |
102 ± 10 |
6.4 ± 1.1 |
21 ± 2 |
30 ± 4 |
15 ± 2 |
262 ± 22 |
18 ± 1.4 |
14200 | 16207 ± 1945 |
Since the accuracy of Pu-239 determination by gamma spectrometry is not great, the concentration of Pu-239 in starting ash was measured by alpha spectroscopy (with preliminary separation of Pu by a standardized procedure) [9], and by total alpha activity measured with a luminescent substance. Results of the plutonium-239 measurements in starting ash using different methods are presented in Table II.
Table II. Plutonium-239 Concentration Measured by Different Methods.
| Method | Gamma Spectrometry |
Alpha |
With Luminescent Substance |
| Bq/g | 14200 |
19637± 1190 |
21657± 1844 |
Volatilization of Radionuclides
In this series of experiments, the loss of radioisotopes to the gaseous phase was measured versus temperature. Starting ash samples of 1-2 grams were heated up to a specified temperature over 10-15 min, and held at this temperature for two hours. After cooling of the equipment, the loss of sample mass was determined. The quartz ampoule (2) and the outlet tubing (4) were filled with the mixture of 1M HNO3 and 1M HCl, and left overnight. Then these solutions were evaporated, and samples for b , g and a spectrometry were prepared. Radionuclide volatility was determined by the ratio of the radionuclide content in the washed out materials to that in the starting sample. The results of these experiments are shown in Table III. As can be seen, the volatility of alpha-active nuclides does not appear to depend on the temperature of ash treatment, whereas that of the beta and gamma-active isotopes clearly increases with the treatment temperature.
Table III. Dependence of Radionuclide Carryover on Temperature of Ash Treatment.
| Variations, % | Treatment temperature, oC |
|||
900 |
1000 |
1100 |
1300 |
|
| Mass | 51 |
56 |
64 |
47 |
| a -Activity | 0.02 |
0.007 |
0.008 |
0.01 |
| b , g - Activity | 2.9 |
11.4 |
14.2 |
19.8 |
Isotopic analysis of the starting ash shows that the temperature dependence of b , g -active isotopes volatility is substantially dominated by the behavior of cesium isotopes. Therefore, a number of experiments were conducted to study the behavior of cesium during heat treatment.
Experiments were carried out as follows:
All samples obtained were analyzed for Cs-137 and Cs-134 content. The quantity of these isotopes detected on the outlet quartz tubing was divided by stages of the experiment proportionally with the cesium collected from the filters and bubblers from each stage. Results of these experiments are presented in Table IV.
Table IV. Cesium Carryover into Gaseous Phase.
| Stages of experiments | Time, min |
Cesium carryover into gaseous phase, % |
| Heating from room temperature to 1300oC | 65 |
13 |
| Holding 1 hour at 1300oC | 60 |
2.4 |
| Holding 1 hour at 1300oC | 60 |
2.1 |
In the final series of experiments, attention was focused on the carryover of alpha-active nuclides. Analytical samples were prepared as follows, after completing the experiments, the quartz ampoule (2) and the outlet quartz tubing (4) were washed and the recovered material was comminuted and digested in a mixture of boiling HF and HNO3. Combined solutions were evaporated, and their gamma activities measured as well as those of the filter (5) and the solution from the bubbler (6). Then alpha-active isotopes were separated from the evaporated combined solutions using standard procedures; these isotopes were deposited on targets by electrolysis, and their alpha spectra measured. Typical alpha spectrum of a sample is shown in Figure 2.
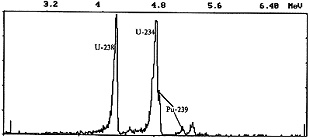
Fig. 2. Typical alpha spectrum of a sample
Results of the experiments on the determination of alpha-active nuclide carryover during heat treatment of ash residues is presented in Table V. In all experiments, the heating ramp to 1300oC was carried out over one hour, then the weighed ash sample was held at this temperature for five hours. After cooling the system, samples for measurements were prepared using the procedure described above; radiochemical yield was 85 %. The measurement time for alpha spectra was varied from 21900 to 25800 s; efficiency of the alpha spectrometer was 47 %.
Table V. Carryover of Alpha-active Nuclides During Heat Treatment of Ash Residues.
Isotope |
Astart. Bq |
Avolatil., Bq |
Carryover % |
Av/Astart. |
U-238 |
668 |
0.288 |
4.3 × 10-2 |
4.3 × 10-4 |
Pu-239 |
36494 |
0.019 |
2.4 × 10-4 |
2.4 × 10-6 |
CONCLUSIONS
As can be seen from the experimental results presented above, the carry-over of alpha-active nuclides in the high-temperature treatment of ash residues under the experimental conditions described is very low. Total carry-over of alpha-active nuclides during heating to as high as 1300oC was less than 0.05%, and it is practically independent of heat treatment temperature within the range of 900-1300oC. Alpha-active nuclide carryover is dominated by the behavior of uranium isotopes. The contribution of plutonium isotopes is about two orders of magnitude lower.
Carryover of b , g -activity is dominated by the behavior of cesium, and occurs substantially during the heating ramp approaching 1300oC, with gradual additional carryover during additional time at. Based on the single set of measurements shown above at varying treatment temperatures, the cesium release appears to be limited up to 900o C, with substantially greater carryover at higher temperatures.
ACKNOWLEDGEMENTS
This work was done under financial support of the United States Department of Energy, through the Mixed Waste Focus Area. The authors would like to express their thanks to Dr. Igor Sobolev, General Director, SIA "Radon", as well as to Dr. Sergey Dmitriev and Dr. Sergey Stefanovskiy for their comments and discussions.
REFERENCES