RELATIONSHIP BETWEEN ACTIVITY PROFILES, SOURCE
AND SOIL TYPES IN SITES CONTAMINATED
AFTER THE CHERNOBYL ACCIDENT
P. Guillou, D. Stammose and J.M. Péres
Institute of Protection and Nuclear Safety
IPSN/DPRE/SERGD BP6
92265 Fontenay aux Roses Cedex France
V. Kashpharov
Institute of Agricultural Radiology
UIAR
Kiev, Ukraine
V. Mironov
Institute of Radiobiology
Academy of Sciences of the Republic of Belarus
IRBAS
Minsk, Republic of Belarus
S. Gordeev
Russian Scientific Practical and Expert Analytical Center - Russian Scientific
Research Institute for Civil Defense and Emergencies
RSPEAC-RSRICDE
Moscow, Russia
ABSTRACT
The present paper is related to the main experimental part of a programme implemented by IPSN on the remediation of sites contaminated after a nuclear accident. It is recognised that one of the key issues for establishing strategies of remediation is our ability to provide accurate assessments of the radiological impact of contaminated sites, through scientific evidence of the real availability of the radionuclides concerned.
In collaboration with eastern Institutes, different sites were selected upon criteria of source term and soil type. The selection process accounted for the differences in the characteristics that may show from one site to another site. One may distinguish four types of sources : low oxidised fuel particles, highly oxidised fuel particles, condensed forms and superposition of oxidised and condensed forms. One may also distinguish four types of soils : podzol, peaty, gley and sandy soils. In 1996, 8 sites were sampled in Ukraine, Bielorussia and Russia. The Cs and Sr activity profiles were measured for each site. Chemical analysis of the various horizons of the soil sampled were also performed.
The results showed that, 10 years after the accident, traces of activity were measured down to 1m for both Cs and Sr. However, more than 90% of the deposited activity still remain in the first 10 cm of all soils, except for the * Prypiat +site (sandy soil) where a fast migration of Sr associated with a phenomena of accumulation is observed. A detailed analysis of all profiles has allowed the pointing out of the main trends :
INTRODUCTION
The optimization of contaminated sites remedial efforts has to carefully account for the management of wastes arising from the various situations encountered in the contaminated environment. It is known that one of the key issues for optimizing mitigation of middle term and long term consequences, after taken emergency counter measures, is the ability to provide accurate assessments of the radiological benefit of site recovery, accounting for residual impacts on sites and storage areas. Since tools for making radiological impact prognose can so far only predict ranges of values with large uncertainties, especially for long term assessments, it is important to reduce these uncertainties significantly and improve the quality of prognoses through scientific evidence of the real availability of the radionuclides concerned. This goal can be attained by the implementation of an experimental programme that are of interest on contaminated sites of the Chernobyl area. This goal can further be reached by the development or improvement of relevant mathematical modelling.
The objectives of the experimental work are to identify the mechanisms responsible for the behaviour of major radionuclides in soils i.e. those of importance during the * post-accidental phase + 137Cs, 90Sr, 241Am and isotopes of Pu. Their behaviour have to be evaluated for groups of different situations. This means that data collection will have to be done on soils of different mineralogy and chemical composition. This should also be the case on sites where the initial deposition of activity have shown various physicochemical forms (fuel particles, or condensed components of fallout).
Through a collaboration with Eastern countries (Belarus, Russia, Ukraine), several sites have been chosen, presenting a combination of the different soil characteristics and sources types. Each of them were sampled at different depths and analysed. In this paper, we have presented only the results obtained from two radionuclides, 137Cs and 90Sr. A first attempt of modelling has been conducted on one site.
Site Selection
A group of eight experimental sites has been selected for studying vertical migration of contaminated material. Samples were submitted for measurements for the purposes of soil contamination characteristic analysis. The eight selected sites are located on three independant states i.e. areas strongly affected by the Chernobyl Nuclear Power Plant (ChNPP) accident : Ukraine, Belarus and Russia. These sites were selected in the near and in the far field to compare scenarios with different types of deposition and a large range of soil characteristics.
Physico-chemical characteristics of deposition were different in the near and far field. Four types of contamination sources were distinguished, corresponding basically to the evolution of the accident and related meteorological conditions : low oxidised fuel particles (Chistogalovka podzol and peat), highly oxidised particles (Prypiat, Zymovishe, Massany), condensed forms (Barki podzol and peat), superposition of highly oxidised and condensed forms (Radin).
The ratio between Sr and Cs that decreased from 1.2 to 0.012, as indicated in Table I, with increasing distance from the ChNPP reflects the diminishing contribution of fuel particules to the total fallout : higher content of fuel particles in the near zone and mainly condensed form in the far field.
EXPERIMENTAL PART
Measurements
Once collected, soil samples were dried, passed through a 1 mm sieve and then their activity was measured. The 137Cs massic activities were determined by g-spectrometry measurements with high purity germanium HPGe detectors. Concerning 90Sr, methods of separation differ according to the Institutes but principles are identical : dissolution of sample, chemical separation, use of tracer or carrier and b counting.
The diameters of samples were measured by Laser Beam Diffraction using a Coulter LS230 granulometer. The sizes of particles measured by this equipment range from 0.04 to 2000 mm. Specific surface areas were determined by the BET (Brunauer Emmet and Teller) method by N2 sorption using a Coulter SA3100.
The measurements of cation exchange capacity (CEC) are realized according to the ammonium acetate method (Jackson, 1964).
Site Description
The description of the sites are summarised in Table I. Typical soils in these areas are soddy podzolic sandy, soddy podzolic sandy loam, soddy gleyed and peaty. Sampling sites were located on mineral soils with different textures (from sandy to gleyed) and on organic soils. The chemical analysis of each of them (expressed as % oxides weight) and some characteristics of soil surface (depth : 0-5 cm) are reported in Table II.
Table I. Site Description, Locations, Deposition, Soil Type
|
Sites |
CHISTOGALOVKA |
CHISTOGALOVKA |
PRYPIAT beach |
ZYMOVISHE |
MASSANY |
RADIN |
BARKI B4-9 |
BARKI B4-10 |
|
Country |
Ukraine |
Ukraine |
Ukraine |
Ukraine |
Belarus |
Belarus |
Russia |
Russia |
|
Distance from ChNPP (km) |
4.4 km WSW |
4.4 km WSW |
4 km NW |
6 km NE |
7 km N/NE |
21 km N |
170 km NE |
170 km NE |
|
Coordinates of sampling points |
51°22'39'' N |
51°22'34'' N |
51°25'08'' N |
51°24'46'' N |
51°31'00'' N |
51°35'10'' N |
52°26'43'' N |
52°26'47'' N |
|
Deposition type |
Low oxidised fuel particles |
Low oxidised fuel particles |
Highly oxidised fuel particles |
Highly oxidised fuel particles |
Highly oxidised fuel particles |
Superposition of highly oxidised fuel particles and condensed forms |
Condensed forms |
Condensed forms |
|
Surface contamination densities |
|
|
|
|
|
|
|
|
|
Massic activities |
|
|
|
|
|
|
|
|
|
90 Sr / 137Cs |
0.60 |
0.59 |
1.20 |
0.78 |
0.34 |
0.12 |
0.012 |
0.023 |
|
Massic activities below 6 cm : |
|
|
|
|
|
|
|
|
|
Annual mean air Temperature (°C) |
6.5 |
6.5 |
6.5 |
6.5 |
7-8 |
7-8 |
5.9 |
5.9 |
|
Annual rainfall (mm) |
566 |
566 |
566 |
566 |
533 |
533 |
529 |
529 |
|
Soil type |
Natural dry meadow formed on soddy-podzolic sandy loam soil, |
Natural wet meadow, formed on peaty soil. |
Soddy podzolic sanfy soil. |
Natural wet meadow, formed on soddy-gleyed soil. |
Soddy-podzolic sandy loam soil. |
peaty soil |
Alluvial, meadow boggy,lightly gleyish soils, peat. |
Soddy alluvial, loamy sand ferriferous acid soil. (clearly distinctive humic-accumulative horizon) |
Table II. Chemical Analyses of Soils (%, Standard Deviation < 0.2 %)
|
|
Soil type* |
SiO2 |
Al2O3 |
Fe2O3 |
FeO |
MgO |
CaO |
Na2O |
K2O |
LOI |
Ctot |
Corga |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHP 0-5 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
CHP 15-18 |
1 |
96.66 |
1.01 |
0.12 |
0.19 |
0.03 |
traces |
0.1 |
0.42 |
1.34 |
0.53 |
0.29 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MAS 0-5 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
MAS 35-40 |
1 |
94.29 |
1.63 |
0.46 |
0.40 |
0.07 |
0.09 |
0.12 |
0.44 |
1.88 |
0.70 |
0.58 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BARP 0-5 |
1 |
79.8 |
2.04 |
1.23 |
1.52 |
0.12 |
0.55 |
0.19 |
0.58 |
14.93 |
7.11 |
4.74 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PRY 0-5 |
2 |
97.06 |
1.21 |
0.22 |
0.19 |
0.03 |
0.07 |
0.10 |
0.51 |
0.65 |
0.17 |
0.11 |
|
PRY 6-9 |
2 |
96.83 |
1.3 |
0.25 |
0.23 |
0.03 |
traces |
0.1 |
0.49 |
0.67 |
0.13 |
0.08 |
|
PRY 12-15 |
2 |
96.37 |
1.64 |
0.41 |
0.10 |
traces |
traces |
0.15 |
0.63 |
0.49 |
0.07 |
0.04 |
|
PRY 18-21 |
2 |
97.5 |
1.35 |
0.23 |
0.13 |
traces |
traces |
0.09 |
0.47 |
0.16 |
0.06 |
0.03 |
|
PRY 55-60 |
2 |
95.85 |
2.09 |
0.37 |
0.20 |
traces |
0.05 |
0.2 |
0.79 |
0.17 |
0.09 |
0.09 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ZYM 0-5 |
3 |
81.3 |
3.23 |
1.12 |
1.08 |
0.14 |
0.39 |
0.27 |
0.74 |
12.22 |
5.34 |
3.85 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHT 0-5 |
4 |
14.06 |
1.15 |
1.64 |
6.69 |
0.19 |
2.40 |
0.05 |
0.21 |
80.02 |
39.69 |
29.43 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RAD 0-5 |
4 |
46.98 |
1.42 |
1.19 |
1.39 |
0.29 |
2.67 |
0.12 |
0.44 |
46.31 |
22.84 |
17.47 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BART 0-5 |
4 |
|
|
|
|
|
|
|
|
|
|
|
|
BART 15-18 |
4 |
70.66 |
5.59 |
8.31 |
4.96 |
0.28 |
0.52 |
0.39 |
1.12 |
10.97 |
2.95 |
1.68 |
*1- soddy-podzolic sandy loam soil; 2- soddy-podzolic sandy soil; 3- soddy-gleyed soil; 4- peaty soil
In mineral soils, pHKCl range from 3.65 to 5.50, organic carbon content range from 0.11 to 4.74 %, and cation exchange capacity (CEC) from 1.1 to 11.0 meq/100g of soil. These mineral sites present a high content of silica (between 80 and 97 %).
In organic soils these characteristics have the following values : pHKCl 4.87 - 5.2, organic matter content 17.5 - 29.5%, CEC 36 - 78 meq/100g of soil, but they present lower content of silica (<50 %).
Fuel Hot Particles Characteristics
The presence of fuel hot particles (FHPs) in the fallout, represented by the uranium oxide matrix with various admixtures, was one characteristic of the Chernobyl accident.The most heavily contaminated land around Chernobyl was within about 20 km of the reactor site where most of the fuel particles fell to the ground. The fuel particles ranged in size from fractions of a micron up to about 150 Fm. Few particles with a diameter in excess of 20 Fm were found beyond 10 km [1]. The basic part of fuel component of Chernobyl fallouts outside the 5 km zone is represented by particles having a median radius of about 2-3 Fm [2].
Within the 30 km restriction zone around the reactor, fuel particles were estimated to account for more than 75% of the total radioactive contamination on the ground [3]. Konoplev et al. [4] reported that in this zone, 80% of the 90Sr was associated with fuel particles compared with 30-40% of the 137Cs. So, its leaching can lead to a significant increase in the migration of Sr. Beyond the 30 km zone northwards and westwards from the ChNPP, excluding the narrow plume directed westward, fuel HPs contain less than 25% of Cs and, consequently, the total destruction of FHPs will not lead to a significant increase in the Cs migration [5]. Southwards and south-eastwards from the reactor, more than 50% of Cs is contained in the fuel component.
Sampling Procedures
The variability of activity distribution profiles in the top layers can reflect several phenomena ranging from experimental artefacts (discrepancies in measurements and sampling methods) to specific site variability, where local characteristics, such as bioturbation and soil stratification can significantly influence nuclide migration. The first step consist of the implementation of intercomparitive exercice between the Institutes engaged in this study. The comparison of sampling methods was done from the survey of 137Cs profiles.
The overall results obtained permit to state that the four different methods of sampling implemented for the determination of the vertical distribution of radionuclides give comparable results with a fairly good level of acceptability on the relative percentage contained in levels of 3 to 5 cm thickness (less than 10% variation). The differences observed in some profiles (Chistogalovska, Barki peat, Massany) are not due to sampling methods but reflect site heterogeneity in the first 5 cm. The intercomparison of sampling techniques permited the conclusion that it was not necessary to adopt a standardised method and that it was possible to use already acquired results by each institute for statistical studies (no bias expected due to the method).
Results and Discussion
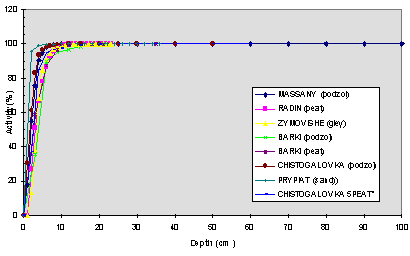
All data on vertical distribution of activity in the soils from the eight experimental sites have been compiled with, so as to identify the major distribution trends and allow comparison of the various profiles between sites. The activity profiles for 137Cs and 90Sr are presented on Fig. 1 and Fig. 2 under the form of cumulated activity percentage for increasing thickness of soil. This cumulative form of representation has been found to be of practical use and allow the determination of the average migration depths and speeds of measured radionuclides in soils, which are useful parameters for the identification of the main differences existing between profiles from the various investigate sites [6]. The average migration depth for one radionuclide corresponds to the thickness of soil containing 50% of its activity; the average migration speed corresponds to former parameter value divided by the time period separating the date of accident from the date of measurement of activity profiles. The average migration speeds have been determined and are given in Table III. It is necessary to bear in mind that the average migration speed from all the profile,V0 (from 0 cm to 1 m), is not a real migration speed because, in the first centimeters, we have to take into account for different phenomena (bioturbation, presence of fuel particles, etc.) which mask the real radionuclides behaviour. To get rid off these first centimeters, we have also calculated the migration speeds from 6 cm, V6. The massic activity values are gathered in Table I.
In a first unrefined approach, we do not see any significative difference was noticed on these profiles, except for Sr on one site. This is in spite of the fact that the studied sites present very different soil characteristics and various deposition types, as showed in Table I and Table II. Ten years after the accident, the most important part of the activity (>96%) was still present in the upper layer of the soils (whatever the site we considered in the first 12 cm for Cs, 15 cm for Sr) excepted for the Prypiat site in which a strong migration of Sr is observed (only 29% in the fisrt 15 cm). These sites are characterized by low migration speeds which are given in Table III : between 0.10 and 0.36 cm/y for Cs and 0.17 and 0.43 cm/y for Sr except for Prypiat 4.41 cm/y. On this latter site, about 44% activity of Sr is present in the 50-60 cm layer. More, for all the sites, traces of activity have been measured down to 1 m for both Cs and Sr.
Table III. Average Migration Speeds (V in cm/y) Calculated from Vertical Distribution of
Radionuclides in Soils and from Some Parameters
|
X |
Sites |
V0 |
V6 |
V6 / V0 |
Soil type * |
pH H2O |
pH KCl |
OM% |
CEC meq/100g of soil |
Clay |
Type of FHP |
Distance |
|
Cs |
PRYPIAT sand |
0.10 |
0.35 |
3.51 |
2 |
5.2 |
4.00 |
0.22/0.11 |
1.10 |
89.95 |
Highly oxid |
4/NW |
|
X |
ZYMOVISHE gley |
0.14 |
0.16 |
1.13 |
3 |
5.5 |
4.48 |
2.21/3.85 |
11.0 |
73.05 |
Highly oxid |
6/NE |
|
X |
CHISTOGALOVKA podzol |
0.16 |
0.15 |
0.97 |
1 |
4.65 |
3.65 |
1.55 |
3.12 |
75.44 |
Low oxid |
4.4/WSW |
|
X |
MASSANY podzol |
0.18 |
0.08 |
0.43 |
1 |
X |
5.5 |
2.9 |
X |
76.76 |
Highly oxid |
7/N-NE |
|
X |
RADIN peat |
0.19 |
0.12 |
0.64 |
4 |
X |
5.2 |
17.5 |
>36 |
72.01 |
Superp Highly oxid and Cond |
21/N |
|
X |
CHISTOGALOVKA peat |
0.22 |
0.47 |
2.07 |
4 |
5.5 |
4.87 |
29.4 |
>78 |
76.30 |
Low oxid |
4.4/WSW |
|
X |
BARKI peat |
0.24 |
0.20 |
0.82 |
4 |
X |
4.96 |
X |
X |
79.50 |
Cond |
170/NE |
|
X |
BARKI podzol |
0.36 |
0.27 |
0.75 |
1 |
X |
4.36 |
4.74 |
14.93 |
80.50 |
Cond |
170/NE |
|
Sr |
CHISTOGALOVKA peat |
0.17 |
0.25 |
1.49 |
4 |
5.5 |
4.87 |
29.4 |
>78 |
76.30 |
Low oxid |
4.4/WSW |
|
X |
RADIN peat |
0.18 |
0.14 |
0.78 |
4 |
X |
5.2 |
17.5 |
>36 |
72.01 |
Superp Highly oxid and Cond |
21/N |
|
X |
MASSANY podzol |
0.22 |
0.09 |
0.39 |
1 |
X |
5.5 |
2.9 |
X |
76.76 |
Highly oxid |
7/N-NE |
|
X |
CHISTOGALOVKA podzol |
0.24 |
0.36 |
1.49 |
1 |
4.65 |
3.65 |
1.55 |
3.12 |
75.44 |
Low oxid |
4.4/WSW |
|
X |
ZYMOVISHE gley |
0.32 |
0.18 |
0.56 |
3 |
5.5 |
4.48 |
2.21/3.85 |
11.0 |
73.05 |
Highly oxid |
6/NE |
|
X |
BARKI peat |
0.38 |
0.27 |
0.71 |
4 |
X |
4.96 |
X |
X |
79.50 |
Cond |
170/NE |
|
X |
BARKI podzol |
0.43 |
0.20 |
0.45 |
1 |
X |
4.36 |
4.74 |
14.93 |
80.50 |
Cond |
170/NE |
|
X |
PRYPIAT sand |
4.41 |
4.31 |
0.98 |
2 |
5.2 |
4.00 |
0.22/0.11 |
1.10 |
89.95 |
Highly oxid |
4/NW |
|
Sr / Cs |
CHISTOGALOVKA peat |
0.77 |
0.53 |
X |
4 |
5.5 |
4.87 |
29.4 |
>78 |
76.30 |
Low oxid |
4.4/WSW |
|
X |
RADIN peat |
0.95 |
1.17 |
X |
4 |
X |
5.2 |
17.5 |
>36 |
72.01 |
Superp Highly oxid and Cond |
21/N |
|
X |
BARKI podzol |
1.19 |
0.74 |
X |
1 |
X |
4.36 |
4.74 |
14.93 |
80.50 |
Cond |
170/NE |
|
X |
MASSANY podzol |
1.22 |
1.12 |
X |
1 |
X |
5.5 |
2.9 |
X |
76.76 |
Highly oxid |
7/N-NE |
|
X |
CHISTOGALOVKA podzol |
1.50 |
2.40 |
X |
1 |
4.65 |
3.65 |
1.55 |
3.12 |
75.44 |
Low oxid |
4.4/WSW |
|
X |
BARKI peat |
1.58 |
1.35 |
X |
4 |
X |
4.96 |
X |
X |
79.50 |
Cond |
170/NE |
|
X |
ZYMOVISHE gley |
2.29 |
1.12 |
X |
3 |
5.5 |
4.48 |
2.21/3.85 |
11.0 |
73.05 |
Highly oxid |
6/NE |
|
X |
PRYPIAT sand |
44.1 |
12.3 |
X |
2 |
5.2 |
4.00 |
0.22/0.11 |
1.10 |
89.95 |
Highly oxid |
4/NW |
*1- soddy-podzolic sandy loam soil; 2- soddy-podzolic sandy soil; 3- soddy-gleyed soil; 4- peaty soil
Nevertheless, the uncertainties related to the activity distributions in the very top layers do not mask the major apparent migration trends for Cs ans Sr in studied soils. If we referred to the Table III, we can give the following decreasing sequences for the migration speeds of Cs and Sr according to the sites studied :
We can notice that Prypiat is a site of a particular interest because on this site there are the lowest migration speed for Cs and the fastest for Sr.

Fig. 1. Vertical distribution of cumulated percentages of Cs137 activity- comparison of selected sites-Sampling October 1996.
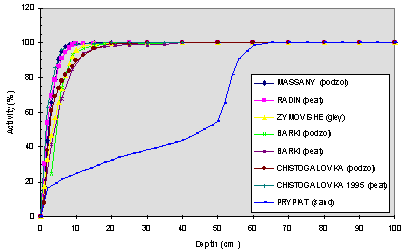
Fig. 2. Vertical distribution of cumulated percentages of Sr90 activity - Comparison of selected sites-Sampling October 1996.
Behaviour of Fuel Hot Particles in Soils and Radionuclide Mobility
Many works indicated that, during the first 2 to 3 years after the accident, the movement of the nuclides associated with FHPs was probably due to mechanical movement of the intact particles (especially in dry podzol soils) as well as to bioturbation [7; 8]. After that period, leaching of radionuclides from the fuel particles became significant. Demchuk et al. [9] produced evidence to show that leaching rather than particle disintegration was the main reason for changes in behaviour of the particles.
Concerning the influence of the presence of FHPs on the migration, we can coarsly divide the sites in two groups depending on the type of sources. The first group corresponds to the remote sites (Barki sites) where the deposition has mainly taken place under condensed forms as indicated in the description of spatial distribution of FHPs : the faster migration speeds, both Cs and Sr (except for Sr in Prypiat), are observed whatever the soil characteristics considered (podzol or peat). The second group corresponds to the near sites where the deposition has been mainly taken place under FHPs. Slower migration speeds obtained are in total agreement with former results [10]. Further more, many field observations and some laboratory experiments have demonstrated the very low mobility of fuel particles in soils. Vertical profiles measured in undisturbed land around Chernobyl showed that even several years after the accident the particles had not reached the 5-6 cm layer [11, 12]. The presence of FHPs is an additional factor in reducing the migration speed of radionuclides.
For this second group, we could expect to observe differences between migration speeds according to the type of deposited FHPs because their velocity of matrix dissolving is different, according to the degree of FHPs oxidation, and therefore the release of radionuclides from fuel particles and their availability for interacting with the soil change. Nevertheless, the migration speeds deduced from observations can not be correlated with the velocity of matrix dissolving. This can be explained in part by the uncertainties related to the vertical distribution of radionuclides in soils and the little differences obtained between migration speeds. We can also note that the portion of destructed fuel particules activity is the highest [13] (from 26 to 95 % in ten years in Prypiat and from 13 to 75 % in Zymovishe for highly oxidized particles compared with Chistogalovka podzol from 9 to 59 % and Chistogalovka peat from 4 to 33 % for low oxidized particles) when the migration speed of Cs is lowest. It become obvious that other phenomena are implicated, directly linked with soil characteristics, in the migration (or immobilization) of radionuclides. This presence of FHPs plays therefore a less and less important role as and when the sites are remote from the ChNPP because the activity deposited under this form gets weaker and weaker.
Soil Characterization and Radionuclide Mobility : Influence of Soil Properties
We have seen that the slow migration of Cs and Sr are explained only in part by the presence of FHPs. The decreasing fraction of radionuclides associated with FHPs is not translated by an important increase in migration speed. Even if the rates of leaching of these radionuclides from particles are significant, their subsequent behaviour is dependent on the type of soil. In fact, physico-chemical characteristics of a soil play a significant role in the behaviour of radionuclides.
When a radionuclide is deposited on (and thus in) the soil, it is likely to undergo certain changes due to its new environment. Species in solution equilibrate with the soil pH, redox status and other solutes, both organic and inorganic reactions occur on the surfaces of soil particles. Once deposited, radionuclides can dissolve as a result of environmental conditions. The effect of these changes determines the retention or the mobility of the radionuclides within the soil. The large number of parameters involved and the interactions between them make it difficult to determine the effect of each single factor.
Considering the presence of FHPs mentioned above, we have chosen to examine data not only on global vertical distribution of activity but also from 6 cm below the surface to a depth of 1 meter in order to get rid of this influence and to try to point out some relationship between the mobility of radionuclides in soils and physico-chemical characteristics of these soils. The migration velocities have been recalculated and we have to bear in mind that the massic activities are generally low or very low below 6 cm as showed in Table I.
Mobility of Cs
The first result already mentioned is the very low mobility of Cs whatever the site This is explained only in part by the association of this radionuclide with FHPs, 30-40 % in the near field and much less in the far field. Nevertheless, we may distinguish a more rapid migration of Cs down the soil profile in peat soils. It becomes evident through the data obtained that we may classify the migration speed according to the soil type and more precisely according to certain characteristics of soils : on the one hand chemical parameters, the organic matter content, capacity of ion exchange and mineralogy, and on the other hand the physical parameter, i.e. granulometry.
As shown in Table III, the sandy site, Prypiat, that has the slowest speed has also the lowest both organic matter contents (< 1%) and CEC (about 1 meq/100g of soil). Then, a group of gley and podzol sites (Zymovishe, Chistogalovka podzol, Massany podzol) characterized by low organic matter and CEC values in that Cs migrates less slowly. In the next group corresponding to the peat soils with high content of organic matter and CEC, Cs migrates slightly more rapidly. In the peaty soils, Cs would not have been hindered so much by the clay minerals. The case of Barki podzol may be explained by differences in forms of Cs present in the near zone and in the remote zone. Exchange and acid soluble forms of Cs were 1.8-3.3 times lower in the near field [14].
The migration of Cs increased with increasing organic matter content. The effect on mobility may be explained by the large CEC of organic matter and the spatial distribution of organic substances around clay particles which prevents adsorption, and subsequent fixation of Cs+ on the clay minerals, and by a weak interaction of Cs+ with organic substances [15]. The adsorption of organic cations on clay minerals takes place by cationic exchange at the clay-solution [16].
So, the organic matter would have a tendency to maintain Cs under exchangeable form [17]. This hypothesis cannot explain all the observed effects. In fact, Evans and Dekker [18] observed positive as well as negative effects of the soil organic matter content on the transfer of Cs. These differences could be explained by different proportions of organic matter components (humic acid, fulvic acid) and/or by different percentages of labile and stabile organic substances. Sobotovitch et al. [19] found a correlation between the intensity of the C-O and C-C bonds in organics compounds and the presence of Cs under water-soluble form. However, a lot of uncertainties remain on the structure of these organic matter (state of evolution) and further studies have to be conducted on the definition of their structure.
A lot of studies [20, 21, 22] indicate that both the clay mineral content and the type of clayey mineral present in soil are factors influencing Cs retention. In the case of Prypiat, the following minerals have been identified in the 6-9 cm layer : quartz (90-95 %), felspar (K felspar 2-5 %, plagioclase 0-3 %) and clayey minerals (mica or illite or interstatified illite/smectite with more than 90% of illite 0-3 % and smectite 0-3 %). If we consider the thin fraction (< 2 mm), results of analysis indicate that more than 80 % in weight of this fraction is composed of quartz. The sorption of Cs is very low on quartz and felspar, not negligeable on micas and important on clayey minerals that have strong cationic exchange capacities. We therefore suppose that, despite a globaly very weak CEC, very little amounts of clayey minerals (illite , smectite,...) are able to fix the quasi-totality of Cs.
In fact, the retention seems to depend not only on mineralogical clay content and type but also on the repartition of different granulometric fractions and particularly on the clay fraction that presents a very high specific surface. As showed in Table IV, all the sites have a high proportion, in surface %, of the granulometric fraction below 2 mm, the sand of Prypiat having a higher proportion of very thin particles. That may be a complementary explanation to the very slow migration speed of Cs on the studied sites, especially Prypiat. The greater adsorption capacity of the smaller soil particles make Cs strongly sorbed in the top soil layer. This hypothesis seems to be confirmed by a stepwise regression [23] showing that particle size account for a significant proportion of Cs variability.
Table IV. Size Distributions and Specific Area of Soils - Depth: 0-5 cm
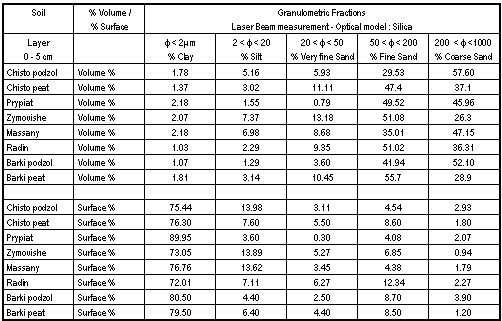
Even after recalculation of Cs velocities, i.e. determination of activity profiles from 6 cm (V6), no significant change appears except for Chistogalovka peat and Prypiat. The rate for adsorption of Cs on most soils substantially exceeds the rate of leaching from the fuel particles. As a result, a stationnary state is established and the distribution of Cs in soils has hardly changed since 1986. For the two exceptions, the behaviour of Cs may be explained in the following way. The chemical characteristics of the top upper layer and the horizons below are quite similar, especially the organic matter content. So, the important factors are probably the presence of FHPs (only until 5-6 cm depth), the types of organic matter and physical differences between horizons. The determination of granulometries and of clay content and type concerning the other horizons would have to choose between the offered possibilities.
Mobility of Sr
Even if Sr migrates slightly faster than Cs, we observe the low mobility of Sr except for Prypiat. This is due in part to the association of this radionuclide with FHPs (80% within the 30 km zone) as above explained.
Total organic matter content and CEC will determine the behaviour of Sr. The Sr mobility generally decreased with increasing amount of organic matter as showed in Table III. The relation between the maximal accumulation of Sr in top horizons of soils and their humus content has already been established for a long time [24].
The interaction of Sr2+ with clay minerals is weaker than for Cs+ whereas the association of Sr2+ with humic or fulvic acids in the organic matter fraction is much stronger. This stronger association may be the consequence of chelation, complexation of the divalent Sr ions at adjacent anionic sites of a humic or fulvic acid [25]. The humic compounds-cations interaction mechanisms lead to the development of easily dissociable complexes (salt type) and of complexes chelate type. The monovalent cations (as Cs+) form salt (humates), only the polyvalent cations (as Sr2+) may lead to chelates [26]. However, in normal organic soils at their natural pH, the presence of soluble chelates or compounds that favours the downward migration was considered to be negligible [27].
The influence of the mineralogy on the fixation of Sr, perhaps to a lesser extent than Cs, has been indicated by Kwaratskhelia et al [24]. Sr bonding in the top horizons and its diminished downward movement is influenced by such chemical substances as gypsum (solonetz) and montmorillonite type minerals (chernozem).
But the differences of migration velocities between sites where the organic matter content is low and high are not very spectacular, except Prypiat obviously. After recalculation of Sr velocities, we observe two types of behaviours, one unexpected. One would expect to observe an increase of migration speed once the influence of FHPs and the most organic horizons is got rid of. The first behaviour that concerns the two Chistogalovka sites confirms this assumption. It can be explained by the presence of FHPs (Chistogalovka peat and podzol) and by a decrease in the organic matter content below the top soil layer (Chistogalovka podzol). The second behaviour shows a decrease of migration velocities, less important for the two peaty sites, Radin and Barki, than for the podzol and gley sites.This decrease is not imputable to the organic matter content because this latter decreases with the depth. So, we have to assume other factors are involved.
The filtration property of soils is a parameter that influences the Sr downward transfer, perhaps in the more important way than organic matter content in certain soils. For the soils corresponding to the second behaviour (i.e decrease of migration velocities after recalculation), the decrease of migration speeds is correlated in all cases with an increase of SiO2 percentage (between 6 and 26 %) and a decrease of the loss of ignition (between 6 and 23 %), as showed by the results of chemical analysis. The consequence is an increase in the soil density in the horizons below the top layer, and a slowing down of the migration speed. But this explanation is insufficient because its doesn't apply in the case of Prypiat where the densities are the highest and quite as well as the SiO2 percentage.
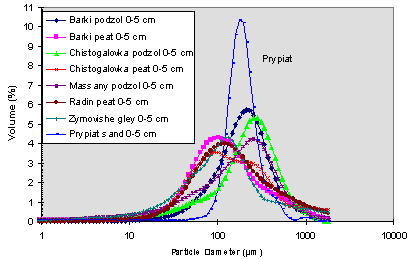
Fig. 3. Grain size distribution in the studied soils differential volume.
The case of Prypiat is very singular because such a rapid Sr migration associated with a phenomena of accumulation have never, to our knowledge, been stated until today after the Chernobyl accident. Nevertheless, similar cases, either fast migrations or accumulation, have already been mentioned but with a different deposition type (atmospheric fallout of nuclear weapons).
So, in this case, the significant parameter would be another factor intervening in the filtration property, i.e. the texture of soil. The sandy textures contrary to the fine or clayey textures confer on soils as particle structure and the absence of cohesion [20]. Prypiat enters in the sandy category what would explain its good filtration properties. Such a noticeable vertical migration of Sr was detected at a 70-100 cm depth in 1960's in podzolic soils [24, 28].
Concerning the accumulation of Sr in the 50-60 cm layer, we may make a suggestion for this phenomena. If we compare the results of size distributions obtained for the layers of Prypiat soil (Table V), significant differences appear between the 55-60 cm layer and all the other layers. In the former case, each granulometric fraction less than 200 Fm has a volume percentage higher than each of the other layers. Globally, these fractions represent more than 98 % in volume whereas its represent between 54 and 81 % for the rest of the vertical distribution with especially a more sensitive difference in the fine sand fraction (89% in the 55-60 cm layer against 49.5-77 % in the other layers) and in the coarse sand fraction (1.8 % in the 55-60 cm layer against 19.2-46 % in the other layers). More, results of specific surface areas in the 0-5 cm (0.4 m5/g) and 55-60 cm (1.4 m5/g) layers confirm also the differences of physical characteristics between these layers and the effect of the surface area increase on the Sr retention. Bearing in mind that one of the processes that governs the vertical migration of radionuclides is the transport, as either solution or suspension form, by water passing through the soil and by diffusion within the soil liquid phase, the change of texture of Prypiat soil becomes fundamental. Generally the distribution of the porosity, and more particularly of the microporosity, depends on the texture [20]. In the present case, the increase of fine granulometric fractions reduces the porosity, meaning the porous volume and pore size distribution, and therefore the movement with infiltrating water and then allows the observed Sr accumulation. Another finding allowed us to confirm this explanation. There are Cs accumulation in the same layer. Indeed, this accumulation is very weak but we have to bear in mind that the activities present in these depths are extremely low (about 0.06 Bq/g or less). The phenomena of accumulation of Sr was observed in 1965 [29] on podzolic soils having about same chemical characteristics than some of the soils we studied.
Table V. Size Distributions and Specific Areas of Prypiat Soil at Different Depth
|
Prypiat |
% Volume / |
Granulometric Fractions |
||||
|
|
|
% Clay |
% Silt |
% Very fine Sand |
% Fine Sand |
200 < f < 1000 µm% Coarse Sand |
|
0-5 |
Volume % |
2.18 |
1.55 |
0.79 |
49.52 |
45.96 |
|
6-9 |
Volume % |
2.27 |
2.58 |
1.34 |
54.11 |
39.7 |
|
12-15 |
Volume % |
2.24 |
0.63 |
0.65 |
77.28 |
19.2 |
|
18-21 |
Volume % |
2.10 |
1.11 |
1.01 |
57.58 |
38.20 |
|
55-60 |
Volume % |
3.20 |
2.66 |
3.04 |
89.33 |
1.77 |
|
65-70 |
Volume % |
2.38 |
1.65 |
2.66 |
66.81 |
26.5 |
|
70-75 |
Volume % |
2.32 |
1.03 |
1.55 |
69.7 |
25.4 |
|
|
|
|
|
|
|
|
|
0-5 |
Surface % |
89.95 |
3.60 |
0.30 |
4.08 |
2.07 |
|
6-9 |
Surface % |
85.9 |
6.5 |
0.6 |
4.9 |
2.1 |
|
12-15 |
Surface % |
90.7 |
1.5 |
0.3 |
6.5 |
1.0 |
|
18-21 |
Surface % |
89.7 |
2.7 |
0.4 |
5.2 |
2.0 |
|
55-60 |
Surface % |
88.61 |
4.0 |
0.75 |
6.57 |
0.07 |
|
65-70 |
Surface % |
88.0 |
3.5 |
0.9 |
6.4 |
1.2 |
|
70-75 |
Surface % |
89.9 |
2.3 |
0.5 |
6.1 |
1.2 |
Modelling of Cs and Sr Migration on Prypiat Site
The objective consists in simulating the evolution of radionuclides vertical distributions in soils. In view of the complexity of describing all mechanisms involved, attemps were made to simulate the basic migration trends observed for Prypiat site by means of a model based on simple hypothesis. The model used to describe such trends, is based on the use of two "global" parameters describing the mobility potential for each radionuclide, one corresponding to the leaching phenomena and one taking into account the presence of FHPs, which stands as an average representation of all the mechanisms influencing migration.
In a schematic manner, the leaching is governed by processus of percolation and sorption. In the analysed configuration, one considers that the leaching depends only on water flow that percolates through the soil and on the strength of sorption.
The annual flow of activity toward the geosphere, Ar (Bq.year-1), will depends on the activity by thickness, At (Bq), and on the annual leached fraction of activity defined as the activity lost during the year divided by the total activity at this year, ALF (year-1). It is expressed as :
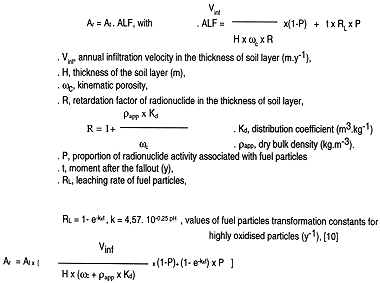
We may deduce from this formula the activity remaining in each of the soil layers, arising only from the loss by leaching, at differentes dates.
The infiltration velocity in the thickness of soil layer corresponds to the effective precipitation during the determined period. This effective rainfall is taken as equal to the half of precipitation waiting to obtain the exact water balance.
The distribution coefficient (Kd) is a parameter generally used to quantify the share of one species between two phases. Kd is usually expressed like ml/g. In our case, the two phases are the solution of leaching and the contaminated soil. This coefficient is defined as the ratio between the specie concentration on the solid phase and the species concentration in the liquid solution. The value of Kd depends broadly on medium conditions (composition of the solution, pH, soil components and physico-chemical characteristics,...). Kd describe the interaction of the radionuclides with the solid materials.
For a sand like Prypiat, the density is closed to 1.4 g/cm3 (from 1.36 to 1.41 g/cm3 according to the layer). The kinematic porosity is chosen equal to 0.4 for a sandy soil [31].
The presence of FHPs and their rate under this form in the upper top layer (0-5 cm) are also taken into account in the model as well as their dissolution. The best fit modelling corresponds to an initial presence of activity under FHPs form equal to about 10%. The dissolution rate introduced in the model was issued from a study of Kashparov et al [10]. The dependence of the constants of FHPs transformation (k, year-1) on the acidity of soil-pHH2O is considered and it corresponds to the case of highly oxidised particles. This dependence makes it possible to predict the dynamics of radionuclides leaching from FHPs. These conditions (pH 5.2 and north west direction from ChNPP) involve a leaching rate of 0.108 y-1. It means that each year 10.8% of the particles are dissolved or that 10.8% of the activity remaining on the site is released in the soil solution.
In a first step, we have focused our work on the most * simple + soil, Prypiat, considering that it is a site of a particular interest because of the lowest migration speed for Cs and the fastest for Sr. Different simulations have shown that four Kd values may be sufficient in a first place to show the great tendencies of Cs migration (Fig. 4). A Kd =1000 ml/g for the 5 first centimeters then Kd values from 30 (10% FHPs) to 160 ml/g (40% FHPs) in the 5-10 cm layer allow the modelling of the migration of cesium while a Kd = 20 ml/g in the 10-15 cm layer and a Kd = 0.5 ml/g down to 15 cm seem to be quite satisfactory. It appears that the cesium behaviour in the upper part of the soil, the first 5 cm, is controlled in a very little part by the source, in part by the very weak amount of clays and the overall by the granulometry of the thinest particles. Further more, we have to bear in mind that the vertical distribution of activity present below 6 cm is very very weak (about 1.9 Bq/g in Prypiat soil). The decrease of Kd values obtained between 0-5 cm and the other layers, meaning a sorption decrease, is correlated with the increase of the Cs migration velocity below 6 cm. The decrease of Kd value obtained between 10-15 cm and the layers below corresponds to a granulometric change. Then, the importance of the granulometry on the Cs behaviour appears obvious.
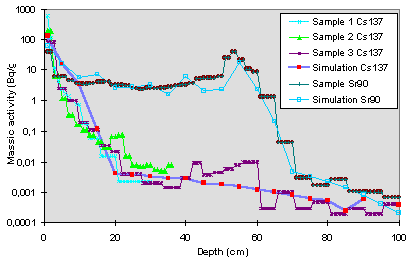
Fig. 4. Comparison between experimental and modelling data vertical distribution of Cs137 and Sr90 activity - Prypiat site.
Like in the case of Cs, different simulations for Sr have shown that four Kd values may be sufficient to obtain a fit between experimental and modelling (Fig. 4). The first value (Kd = 50 ml/g) high enough for a sandy soil corresponds to the first centimeter and may be correlated with the presence of FHPs. The second (Kd =2 ml/g, 1-15 cm) and third (Kd =0.5 ml/g, 15-50 cm then 60-100 cm) values are low and compatible with a sandy soil in which cations are poorly retained. The difference between these two latter values is due to granulometric differences just like in the case of Cs. The fact that there are no changes of Kd values betwen 1-5 cm and 5-15 cm, contrary to the Cs behaviour, is related to the fact that there are no modification of the Sr migration velocity below 6 cm. The value (Kd = 0.5) obtained below the accumulation horizon confirms the particularity of this layer. The Kd value (25 ml/g) obtained between 50-60 cm depth is necessary in order to have a good fit between measurement and calculations. This value corresponds to the phenomena of Sr accumulation discussed above and is correlated with a change of granulometry.
CONCLUSION
In a first unrefined approach, we have notice no significative difference on these profiles, except for Sr on one site, in spite of the fact that the studied sites present very different soil characteristics and various deposition types. Nevertheless, the uncertainties related to the activity distributions in the very top layers do not mask the major apparent migration trends for Cs ans Sr in the studied soils.
Concerning the FHPs, their presence plays a less and less important role as and when the sites are remote from the ChNPP because the activity deposited under this form gets weaker and weaker.We can even note that the part of destructed fuel particules activity, and therefore released from fuel particles then available for interacting with the soil, is the highest when the migration speed of Cs is the lowest : physico-chemical characteristics of the soil play a significant role in the behaviour of radionuclides.
The study between some parameters and the migration speed of Cs have been showed lower migration speeds of Cs with increases in the fine particles and perhaps increases with increases in the organic matter content and CEC. But, this latter hypothesis will have to be checked because positive as well as negative effects of the soil organic matter content have been observed on the transfer of Cs. As a result, the rate for adsorption of Cs on most soils substantially exceeds the rate of leaching from the fuel particles and a quasi-stationnary state is established.
The behaviour of Sr appears largely determined by a group of parameters : its rate of leaching from the particles, the total organic matter content, the cation exchange capacity, and the granulometry-porosity of soils. The Sr mobility generally decreased with increasing amounts of organic matter and cation exchange capacity. The filtration property of soils is also a parameter that influences the Sr downward transfer, perhaps in the more important way than organic matter content in some soils.
Prypiat is a site of particular interest because on this site there are the lowest migration speed for Cs and the fastest for Sr, such a rapid Sr migration associated with the phenomena of accumulation have not, to our knowledge, been stated to date after the Chernobyl accident. The Cs behaviour may be explained by the lowest contents of both organic matter and CEC, and the highest percentage of the surface. In the case of Sr behaviour, the significative parameter would be the sandy texture of soil that would explain its good filtration properties. The accumulation of Sr in the 50-60 cm layer corresponds to the increase of fine granulometric fractions and to a reduction of the porosity, and therefore to a decrease of the movement with infiltrating water.
The interaction of soils and radionuclides is rarely controled by one dominant factor. Generally, the reactions and behaviour observed in the field are a complex interplay of the physico-chemical properties of the radionuclides and those of the soil. In order to confirm all the advanced explanations, it will be necessary to conduct a lot of studies on the evolution of the fuel component, dissolution and/or migration of fuel particles in the soils and on the respective importance of physical parameters (type and content of mineral clays, specific surface areas, porous volumes and pore size distributions, granulometric distributions) and chemical parameters (type and content of organic matter, content of CEC). Further more, we have foreseen to confirm all these results by statistical measurements on vertical distribution of activity in soils. In a second phase, it will be necessary to introduce the obtained correlations in the model so as to determine which parameters are significative, the aim being to predict reliably how 137Cs and 90Sr will interact with soils and how these interactions will respond to changes in the condition of the soil.
REFERENCES