SPECIFIC DECONTAMINATION PROBLEMS OF RADIOACTIVE
WASTE WATER REMEDIATED BY METAPURE
Ilzycer, I. Gilath, A. Mey-Marom, Y. Aleq, O. Even and H. Zafrir
SOREQ, Nuclear Research Center, Yavne 81800, ISRAEL
Tel. 972 - 8 - 9434134 Fax 972 - 8 -9434403
Tel Or and N. Cohen, The Hebrew University of Jerusalem
Faculty of Agriculture, Rehovot, ISRAEL
Tel. 972 - 8 - 9481262 Fax 972 - 8 -9467763
ABSTRACT
Metapure biomass, based on a dry plant, has remarkable selective
binding and concentrating ability for radioactive and heavy metal ions from waste water,
in a broad range of concentration (ppm to below ppt) and over a wide range of pH: 2-12.5.
Metapure is a green product and is easily incinerated at low temperature to heavy metal
enriched ashes, reducing the waste volume for disposal up to 1/10.
Metapure biomass was found to have almost no affinity for Na ions,
while retaining its selective absorbing properties for other metal ions, and consequently,
proved to be quite efficient in brine solutions.
In this work three specific problems of decontamination were studied:
removal of radioisotopes traces in real waste water at pH ³ 12
, cesium removal from high sodium nitrate content solution, and fission products
decontamination from high hardness water. The incineration of used Metapure at 500°C, was also investigated resulting in the reduction of the weight
by a factor of 10 without any loss of the absorbed radioactive isotopes.
INTRODUCTION
Metapure, a patented material based on a dried plant (biomass), binds
and concentrates radioactive and heavy metal ions from water solutions in a broad range of
concentration (ppm to below ppt) and over a wide range of pH: 2-12.5. In real tests,
Metapure was found to bind and concentrate metal ions in solution at ultra low
concentrations, much below ppt. Metapure biomass is suitable for remediation of nuclear
industry waste water from radioactive and toxic metal ions.
In our former paper "Removal of radioactive and heavy metal ions
from waste water using Metapure" (WM’97), the binding property of Metapure was
demonstrated for model solutions containing radioactive ions at low concentrations. The
clean up efficiency for these model solutions was close to 100%.
It was also shown that unlike strong nuclear grade cation exchanger,
Metapure biomass has almost no affinity for Na ions, while retaining its selective removal
properties for other metal ions, and consequently, proved to be quite efficient in brine
solutions. For a solution containing 20 g/l NaCl (comparable to sea water) and 2 ppb
Cs-134, the Cs-134 removal was 66% for 1000 ml feed solution and 10 g Metapure dry biomass
column.
METHODOLOGY
Solutions containing radioactive isotopes as metal ions were prepared
in the laboratory, when not using real solution.
The experimental laboratory set-up consisted mainly of a vessel for the
feed solution, a peristaltic pump to flow the feed solution through a packed column with
Metapure, a vessel for the effluent, a NaI nuclear detector for activity measurements of
solution concentration above ppt range and HPGe nuclear detector for activity measurements
of solution concentration below ppt range.
The laboratory column was 1.5 cm diameter and 15 cm length and
contained 5 g of dry biomass. The flow rate experimented were from 2-120 ml/min. For
isotopes concentration above ppt range and during the flow experiments through the
biomass, the column was placed in front of the NaI detector for continuous real time
measurement of radionuclides uptake in the column. The metal ion concentration was
determined by activity measurements on feed and effluent solutions as well as the metal
ion uptake on the Metapure column, allowing calculation of material balance.
Used Metapure filters, were incinerated at 500°C.
The biomass was pulled out from the laboratory column and disposed in a porcelain
crucible. The porcelain crucible was placed inside an incineration furnace and the
temperature was raised slowly up to 500°C. The temperature was
maintained at 500°C, for about one hour to insure complete
incineration. The resulting ashes were acid digested with HNO3 prior to
dissolution in water. The isotopes activities were measured before and after incineration
for comparison.
RESULTS
1. Removal of radioactive isotopes from highly alkaline effluents:
Radioactive isotopes present at very low concentrations in the
Soreq reactor cooling water, are removed by passing the water through a mixed bed ion
exchange resins. The regeneration of these resins results in highly alkaline effluents, at
pH around 12.5, containing mostly fission products at concentration much below ppt (10-5-10-2
ppt). Decontamination of these effluents was attempted using Metapure. The biomass of
plant origin, was resistant above expectations in the harsh alkaline feed. One liter of
the effluents, containing the radioactive isotopes, was passed through a 5 g Metapure
column, at a flow rate of 4 ml/min. The activity of the isotopes in the initial solution
(input solution) and in the output solution from the Metapure column, was measured on 200
ml samples, using a HPGe detector at constant geometry. The concentration of the fission
products was calculated using the specific activity of the isotope. The results are
presented in Table I:
Table I. Cleanup of Radioactive Isotopes Traces from Highly Alkaline
Effluents
| Isotope |
Cs-137 |
Co-60 |
Ba-140 |
Ru-103 |
Zn-65 |
| Input solution |
|
|
|
|
|
| Activity nCi |
1.135 |
1.43 |
2.14 |
0.283 |
0.93 |
| Concentration ppt |
6.2 10-2 |
6.4 10-3 |
1.5 10-4 |
4.5 10-5 |
|
| Output solution |
|
|
|
|
|
| Activity nCi |
0.352 |
0.526 |
ND |
0.131 |
ND |
| Concentration ppt |
1.9 10-2 |
2.3 10-3 |
ND |
2.1 10-5 |
ND |
| Removal |
69% |
63.2% |
100% |
53.7% |
100% |
*ND: not detected
The above results demonstrate the efficiency of Metapure for extreme
alkaline solutions and were used to design a Metapure filtering system to remediate the
effluents.
2. Decontamination of radioactive solutions containing very high
concentration of NaNO3
High concentrated solutions of sodium nitrate, are often
found in nuclear wastes. Metapure was tested for NaNO3 concentration range of
17 - 187 g/l and containing 2 ppb of Cs-134.
The uptake of Cs decreases linearly with increasing NaNO3
concentration. For 17 g/l NaNO3 solution (6,700 ppm Na) and 5 g Metapure per
1000 ml feed solution, the uptake of Cs-134 was 22.7%. By increasing the amount of
Metapure or for solution concentration less than 17g/l NaNO3, the yield of
removal is increasing. For this solution (17 g/l NaNO3), the Cs concentration
is by six order of magnitude lower than Na concentration.
This facts demonstrate the high selectivity and affinity of Metapure.
The removal of Cs-134 for different NaNO3 concentrations is shown in Table II
and Fig.1.
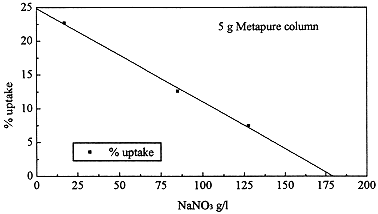
Fig. 1. Cs uptake as a function of NaNO3 concentration.
Table II. Cs-134 Uptake (2 ppb initial concentration)
NaNO3 g/l |
% uptake |
17 |
22.7 |
85 |
12.6 |
127.5 |
7.5 |
187 |
0 |
3. Removal of fission products from high hardness water:
In order to evaluate the efficiency of Metapure to clean up
radioactive isotopes at very low concentration in tap water, fission products isotopes
were produced by neutrons irradiation of 0.4 mg U-235 (purchased from Isotope Products
Lab., Burbank, USA), at the Soreq NRC reactor and then diluted tap water. The tap water of
high hardness contains 68 ppm Ca, 12 ppm Mg, 31 ppm Na and 2 ppm K. One liter of the tap
water solution, containing the radioactive isotopes traces, was passed through a 5 g
Metapure column, at a flow rate of 4 ml/min.
The activity of the isotopes in the initial solution (input solution) and in the output
solution from the Metapure column, was measured on 200 ml samples, using a HPGe detector
at constant geometry.
The concentrations of the fission products were calculated using the
specific activity of the isotope. The fission products concentrations were by 9 order of
magnitude lower than the ionic composition of the tap water. The removal efficiency, shown
in Table III, is close to 100%.
Table III. Cleanup of Fission Products Isotopes at Very Low
Concentration in Tap Water
Isotope |
Input solution
(ppt) |
Output solution (ppt) |
Removal % |
Nd-147 |
1.0 10-4 |
5.5 10-4 |
100% |
Ce-141 |
3.1 10-3 |
5.4 10-5 |
98% |
Ru-103 |
4.9 10-3 |
1.7 10-3 |
64% |
Ba-140 |
1.5 10-3 |
ND |
100% |
Zr-95 |
8.0 10-3 |
1.7 10-4 |
98% |
Nb-95 |
2.3 10-3 |
4.5 10-5 |
98% |
La-140 |
5.5 10-4 |
1.5 10-6 |
98% |
4. Incineration of Metapure
Metapure being of plant origin can be incinerated at low
temperature to heavy metal enriched ashes, reducing the weight of waste for disposal by a
factor of 10. Some of the Metapure filters, used for the removal of fission products from
high hardness water, were incinerated at 500°C. The isotopes
activities were measured before and after incineration. The results shown in Table IV,
demonstrated that all the radioisotopes were recovered without any loss.
Table IV. Incineration of Metapure Filters
| |
Filter no 1 |
Filter no 2 |
Isotope |
Activity before incineration (nCi) |
Activity after incineration (nCi) |
Activity before incineration (nCi) |
Activity after incineration (nCi) |
Ce-144 |
3.2 |
3.05 |
2.71 |
2.80 |
Ce-141 |
30.0 |
30.5 |
29.0 |
29.5 |
Ru-103 |
9.1 |
10.3 |
8.7 |
9.4 |
Ba-140 |
4.62 |
4.81 |
4.8 |
4.9 |
La-140 |
17.6 |
21.2 |
17.0 |
19.9 |
CONCLUSIONS
The efficiency of Metapure to decontaminate fission products and heavy
metal radioactive trace elements from nuclear waste waters was demonstrated for extreme
conditions of alkalinity and cation concentrations. Incineration of the biomass reduces
considerably the volume for waste disposal.
BACK
