DECONTAMINATION OF ELECTROMECHANICAL PARTS BY
THE SONATOL PROCESS : II - RESULTS
R. Kaiser and C.S. Yam
Entropic Systems, Inc.
Winchester, MA 01890
Patty Drooff and Paul Jones
Boston Edison Company
Pilgrim Nuclear Power Station
Plymouth, MA 02360
ABSTRACT
ESI’s Sonatol¸ process, initially developed for the cleaning of inertial guidance instruments parts, has been successfully applied to the nondestructive decontamination of equipment. The cleaning medium used in this process, a solution of a high molecular weight fluorocarbon surfactant in a fluorocarbon solvent, does not present a hazard to the atmospheric ozone layer..The Sonatol Nuclear Decontamination System (SNDS) was installed in June 1997 at the Pilgrim Nuclear Power Station of the Boston Edison Company . Operating results obtained during initial trials will be discussed. In particular, a wide variety of electronic equipment was decontaminated, including a desktop computer, a computer monitor, voltmeters, micrometers, a torque wrench and wire cutters. These items were not only decontaminated to free release levels, but were still functioning after decontamination.
INTRODUCTION
Particles of micrometer size or smaller are generally observed to adhere tenaciously and non-specifically to other solid surfaces, and can not be removed by simple mechanical means. There is a large body of physical evidence that suggests that their adhesion is due to secondary valence forces between the particles and the substrate 1. The presence of such fine particles can result in a significant deterioration in product quality and yield in many precision manufacturing operations of commercial interest. The removal of unwanted fine particles from solid substrates is a problem that has been recognized for several decades 2,3 and is of continuing importance 4.5.6 .
The traditional methods of removing unwanted particles have had a major environmental impact. Chlorofluorocarbons (CFC's) and methyl chloroform (1,1,1 trichloroethane) (TRIC), which have been widely used for particulate removal in precision cleaning operations, were two of the major sources of atmospheric chlorine, which was responsible for the devastation of the ozone layer. The Montreal Protocol agreements resulted in the phasing out of CFC-113 (trichlorotrifluoroethane) and TRIC, and these products are no longer manufactured in the U.S.
In particular, CFC-113 and TRIC were used extensively in the manufacture and maintenance of reliable inertial guidance system gyros. Because of the small clearances involved, inertial guidance instrument manufacturers try to eliminate all foreign particles larger than 5 mm from the instrument component parts. Under the auspices of a SBIR (Small Business Innovation Research) program (AF 91-186) sponsored by the Ballistic Missile Organization of the U.S. Air Force, Entropic Systems, Inc. (ESI) was able to demonstrate that its ENTROCLEANTM enhanced particle removal process removed small particles from solid surfaces much more effectively than traditional CFC-113 based processes. In this process 7.8, the part(s) to be cleaned are sonicated in a flowing stream of a dilute solution of a high molecular weight fluorocarbon surfactant in an inert perfluorocarbon (PFC) liquid, then rinsed with pure carrier liquid to remove residual surfactant, and then dried. The perfluorinated liquid solutions used in the process have zero ozone depletion potential, are nontoxic, nonflammable and are generally recognized as nonhazardous materials. While there is some concern about their global warming potential, these materials have been accepted by the EPA as an environmentally acceptable means of cleaning highly complex geometric configurations that must meet stringent particulate cleanliness standards.
APPLICATION TO THE NUCLEAR INDUSTRY
In the nuclear industry, facilities and their components inevitably become contaminated with radioactive materials. While contamination may be due to a chemical reaction between radioactive process materials with the materials of construction of these facilities and components, it is often due to the deposition of thin films of fine adherent radioactive particles that are not chemically bonded to the substrates, especially in the case of accidental discharge.
Design and operational procedures contribute to minimize these effects, but decontamination is a necessary process in reducing radiation levels in the working environment. Whereas facilities are occasionally cleaned either for reuse or decommissioning, components from active areas commonly require decontamination for reuse or maintenance operations on a much more frequent schedule.
Decontamination processes are similar to traditional surface cleaning processes which involve the removal of unwanted nonradioactive surface contaminants, except as to the types of contaminants and, to a certain extent, the degree of removal. Nuclear decontamination requires an essentially complete level of contaminant removal, a level of cleaning perfection which is required by only select manufacturing operations which are very sensitive to foreign contaminants, such as the inertial guidance industry.
Chlorofluorocarbons, such as trichlorotrifluoroethane (CFC-113), have been of particular interest to the nuclear industry for they offer the ability to clean electrical components without damage, and can clean product contaminated material without the risk of criticality9. In particular, high pressure spraying of CFC-113 has been found to be an effective means of removing radioactive particulate contamination from devices, including electrical equipment and hardware 10.
Decontamination processes based on CFC's are now obsolete and can no longer be used because of environmental concerns, as mentioned above. As a consequence, the only viable method of reclaiming radioactively contaminated electronic and similar sensitive high value equipment was no longer available to the nuclear industry.
Nuclear contamination is inherently a surface phenomenon. Much nuclear waste is the result of the deposition of radioactive particles on nonradioactive substrates. The presence of radioactive particles is responsible for all "smearable" contamination, and, if the radioactive particles are small enough, for some of the fixed contamination. Since radioactivity does not influence the physical chemistry of particle adhesion, ESI believed that the ENTROCLEANTM process should be just as effective in removing radioactive particles from parts as nonradioactive particles.
Under the auspices of a U.S. Nuclear Regulatory Commission (USNRC) SBIR program, ESI was able to initially develop, and then demonstrate, the application of the ENTROCLEANTM process as a means of removing radioactive particles from large, and therefore costly to dispose of, contaminated objects of high inherent value. The suspended radioactive particles would then be removed from the process liquid by filtration, so that the liquid could then be reused. The radioactive particles from the decontaminated parts accumulate on the filter. The filter and the captured particles are periodically disposed of as radioactive waste. The perceived advantages of the proposed process were:
PROCESS DEVELOPMENT PROGRAM
Process development studies were performed at the Nuclear Research Laboratory of the Massachusetts Institute of Technology, Cambridge, MA. The results of the process development, which have been previously published11 are summarized as follows:
a. The fluorocarbon surfactant solutions used as working media in the process survived exposure of to up to 10 Mrad doses of gamma rays, and are considered sufficiently radiation resistant for use as decontamination media.
b. Ultrasonic cleaning in perfluorinated surfactant solutions was found to be an effective method of removing radioactive iron (Fe 59) oxide particles from circuit boards.
c. Significantly higher levels of decontamination were obtained by sonicating in a flowing fluorocarbon surfactant solution than by using CFC-113 or a pure PFC under similar conditions. The decontamination factors (DF) obtained after one hour with different media under otherwise similar experimental conditions are summarized below:
| Process Liquid | DF | Error |
| CFC-113 | 64 | 5% |
| PFC [Vertrel 245 (DuPont)] | 93 | 8% |
| PFC [PF-5070 (3M)] | 250 | 15% |
| PF-5070 + 0.3 wt-% surfactant | 1213 | 31% |
d. The radioactive particles removed from the circuit boards and suspended in the process liquids were quantitatively removed by filtration through a 0.22m membrane filter, with a filtration efficiency of 99.5% per stage.
e. These process liquids used in the process can be recycled for further use by filtration and distillation.
f. All the circuit boards used in the test program were still functional after the cleaning experiments. Neither the process liquids nor the maximum level of ultrasonic power used in the tests had any deleterious effects on the boards or the circuit components.
USNRC DEMONSTRATION PROGRAM
In order to implement the findings of the process development study on a realistic manufacturing scale, Entropic Systems, Inc. designed and built a commercial scale decontamination system, named the "Sonatol Nuclear Decontamination System" (SNDS), which can physically handle the wide variety of contaminated parts that can be found in commercial nuclear power plants.
Performance Criteria
The first major objective of the program was to design and build a decontamination system that would meet the following criteria:
The second major objective was to demonstrate the operation of this system under realistic user conditions.
System Design
The design of the SNDS is based on the following parameters:
The system operating stability is monitored by temperature and pressure devices to ensure the inherent safety. In addition, the SNDS is configured with active and passive elements incorporated to maximize solvent containment, thereby minimizing solvent loss.
The SNDS can be operated either in a fully automated or manual mode. The operator is guided through the operation of the system by a series of self-explanatory commands displayed on a key-pad screen.
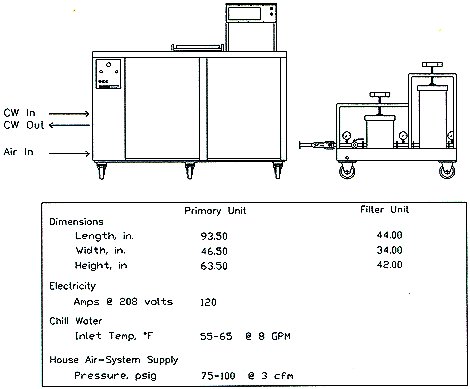
Figure 1 presents schematic representations of the primary cleaning system and the associated filter module, as well as utility requirements. System layout of the SNDS is presented in Figure 2.

Figure 1. SNDS Installation Drawing
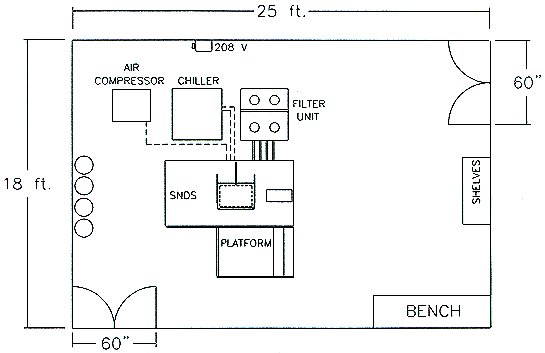
Figure 2. SNDS System Area Layout
The SNDS design incorporates the following major system elements, as outlined in the flow schematic presented as Figure 3
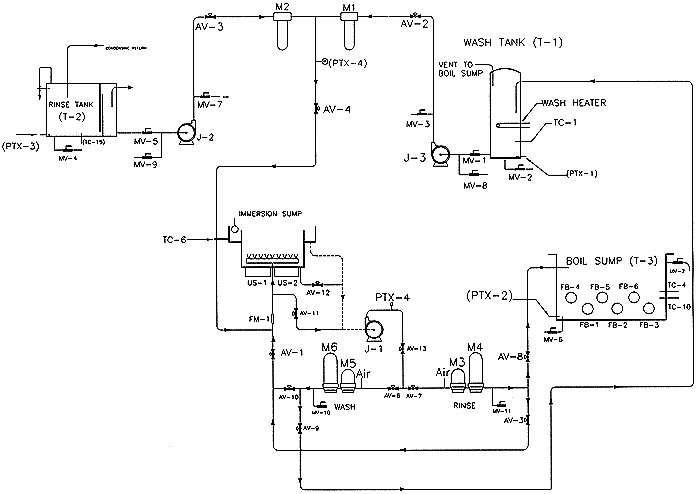
Figure 3. SNDS System Flow Schematic
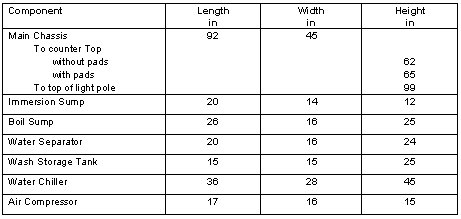
Dimensions of the SNDS and the principal tanks are given in Table I
Table I. Dimensions of the Principal Components of the SNDS
Liquid capacity of the SNDS and of the principal tanks are given in Table II.
Table II. Fluid Capacity of the SNDS
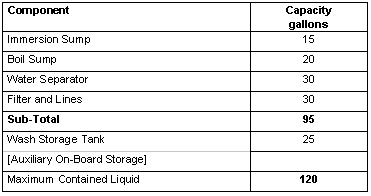
After the cleaning process, the immersion sump is then drained through filters M-5 and M-6 into the wash solution storage tank. The major difference between the wash loop and the rinse loop is the absence of a boil sump.
Process Description
The SNDS has the ability to support parts cleaning with either one of two independent process loops, the wash loop and the rinse loop. These two loops connect at the immersion sump sparge inlet. This arrangement allows a variety of chemistries to be used in the SNDS.
Rinse Loop : Refering to Figure 3, the principal components of the rinse loop consist of:
Starting with the rinse storage tank, pure rinse liquid is drawn from the tank by the rinse pump J-2. The rinse liquid is forced through the rinse pre-filter, M-2, which remove particles greater than a desired size, into the immersion sump. In the immersion sump, the liquid is brought into contact with the parts that are being cleaned. The rinse pump, J-2, stops when the immersion sump is filled. The rinse liquid is then recirculated through the sump and rinse filters, M-3 and M-4, by the main circulation pump, J-1. Under the combined action of fluid flow and ultrasonic agitation, contaminants are removed from the parts by the rinse liquid. Removal (or cleaning) mechanisms include dissolution and mechanical suspension of contaminants. After the rinsing process, the contaminated cleaning liquid then drained from the immersion sump into the boil sump through the rinse filters M-3 and M-4. In the boil sump, the rinse liquid is distilled, separating the contaminants from the recycled rinse liquid.
Wash Loop : The principal components of the wash loop consist of:
Starting with the wash solution storage tank, wash solution is drawn from the tank by the wash solution pump, J-1. The wash solution is forced through the wash solution pre-filters, which remove particles greater than a desired size, into the immersion sump. In the immersion sump, the liquid is brought into contact with the parts that are being cleaned. The wash pump, J-3, stops when the immersion sump is filled. The wash liquid is then recirculated through the sump and the wash filters, M-5 and M-6, by the main circulation pump, J-1. Under the combined action of fluid flow and ultrasonic agitation, additional contaminants are removed by enhanced mechanical lifting.
Drying: In addition to the above liquid immersion operations, parts are also vapor dried in the SNDS. This is accomplished by superheating the vapor zone above the immersion sump to a temperature higher than that in the boil sump. Allowing solvent wet parts leaving the immersion sump to dwell in this superheated zone, their temperature will rise above the boiling point of the solvent. This will cause drag-out liquid to evaporate from the parts, and thus minimize solvent losses when the parts are removed from the system. The liquid that is evaporated in the superheat zone is recovered by the cold condensing coils which define the top of the vapor space.
Decontamination Tests
The construction of the SNDS was completed in the first quarter of 1997. The system was functionally tested before its delivery to the Pilgrim Nuclear Power Station (PNPS) of the Boston Edison Company (BECo) in June 1997. The system was installed and operated for purposes of demonstration in the Station Services Trash and Laundry Building within the Radiological Controlled Area (RCA). Data were obtained by both ESI and BECo staff. The system was operated for purposes of demonstration from July 8, 1997 to the end of the program (August 31, 1997).
Decontamination Results
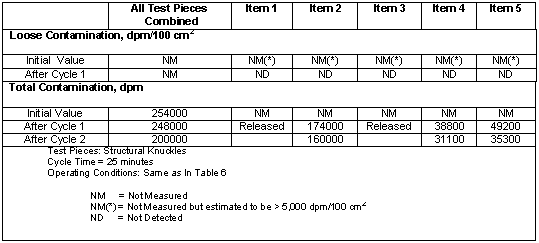
Parts Tested: BECo personnel collected a variety of contaminated parts for decontamination in the SNDS. These parts consisted of electronic equipment, such as computers, survey meters, and electronic instruments; precise mechanical tools, such as torque wrenches and micrometers; electrical equipment, such as motors and potentiometers, as well as miscellaneous tools and machine components. These items were specifically selected for these tests to salvage the parts for reuse. Except for five scaffolding knuckles, no loose contamination was found on any of the accessible surfaces.
A total of thirty-one parts were tested in the SNDS, as listed in Tables III and IV. These varied from less than 1 cm3 (a vacuum test filter) to more than 1 ft3 (computer monitor) in overall size. They were all geometrically complex in shape. Materials of construction were varied, including metals, ceramics, plastics, and elastomers. The level of cleanliness of the as received parts ranged from very clean to very dirty. Some parts, such as the vacuum tester components (Items 24, 25 and 26), were freshly contaminated, while others, such as the electric motors and sensors, had been contaminated for years. The knuckles discussed in Table IV range from being nearly new to being visibly old and corroded.
Table III. Results- Total Contamination Level (DPM) as Function of Cleaning Time
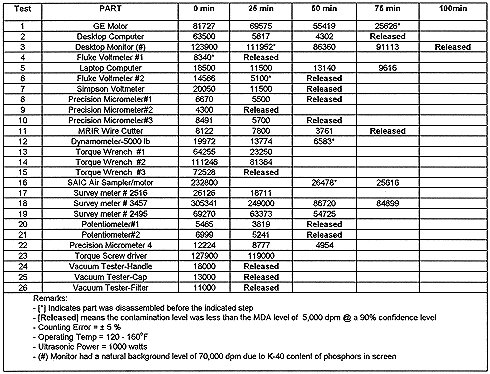
Table IV. Results- Removal of Loose Contamination
Processing Conditions: During the evaluation trials, the system was operated mainly in the manual mode in order to have the flexibility to vary process conditions in order to define optimal processing times under the optimized operating conditions. Parts were subjected to one or more cleaning cycle of 25 minutes duration. Some of the parts were partially disassembled prior to cleaning or between cleaning cycles, or rotated between cycles, to improve solid-liquid contact.
Assessment of Decontamination Levels: The level of radioactivity of individual parts was measured before and after processing in the SNDS by a BECo Radiation Protection Technician with a SAM 9 detector manufactured by the NE Technology. This instrument is normally used to evaluate the level of contamination for parts to be freely released from the RCA. The free release is set at an MDA (Minimum Detectable Activity) of 5000 dpm at a 90% confidence level.
Decontamination Results
Contamination levels as a function of cleaning time for the twenty-six parts tested are presented in Table III. All processed parts were found to be functionally operational after decontamination.
Discussion of Results: The most important finding is that 17 of the 31 parts tested were decontaminated to below free release levels. Of these parts, eight were decontaminated in one twenty-five minute cycle, six required two cycles, and three required three or more cycles. For the five knuckles, loose contamination was reduced from an assumed level of 5,000 dpm/100 cm2 or greater to below detection levels in one cycle. Decontamination factors for parts that were released ranged from more than 1.1 to more than 12. Significant decontamination factors were also obtained for parts that were not decontaminated to free release levels. These ranged from 1.1 to 11.4.
The following physical properties were found to impede the decontamination of parts:
The purpose of the study was to assess the stand-alone decontamination effectiveness of the Sonatol process. It is fairly evident that a number of additional parts could have been decontaminated to free release levels by combining other decontamination means with the Sonatol process.
If mixed waste generation is not considered to be a primary issue, ESI has used alternate cleaning media that effectively remove hydrocarbon based oils and greases in non-nuclear applications. The perfluorocarbon base liquids currently used in the SNDS could be replaced by one of these alternate cleaning media. This would result in lower residual contamination levels of greasy and grimy objects such as the motor listed as Item 1.
CONCLUSIONS
The Sonatol process is able to decontaminate a wide variety of previously non-decontaminable parts without impairing their functional characteristics. Most of the parts treated will be sufficiently decontaminated to allow their release from the radiological control area.
The process appears to be especially well suited for the decontamination of sensitive electronic equipment such as computers, and electronic instruments which would be damaged by any of the other decontamination methods currently in use.
The results obtained indicate that the process is especially effective in cleaning parts that were recently contaminated. This indicates that the SNDS would be a very valuable tool for the decontamination of parts and instruments during an outage when significant levels of both utility and contractor owned equipment become contaminated.
The Sonatol process should not be considered to be a general answer to all contamination problems, but rather, it should be considered as a new and valuable tool that can enhance the overall decontamination capabilities of a nuclear facility.
REFERENCES