SOME OPPORTUNITIES OF FEASIBLE MANAGEMENT
OF RADIOACTIVE ASH RESULTED FROM LARGE
SCALE VALORIZATION OF CONTAMINATED
BIOMASS IN CHERNOBYL REGIONS
Alexandre Grebenkov
Institute of Power Engineering Problems
Sosny, Minsk 220109, Belarus
Phone # 375 17 246 7542; Fax # 375 17 246 7055; E-mail: greb@sosny.bas-net.by
Vjacheslav Zabrodsky
Institute of RadioEcological Problems
Sosny, Minsk 220109, Belarus
Paul Kalb
Brookhaven National Laboratory
Upton, NY, USA
ABSTRACT
Wood from a forest contaminated as a result of Chernobyl Accident sooner or later occurs either in domestic stoves or industrial boilers. Since the time of the Accident more than 200 thousand tons of radioactive ash were collected and dispersed in Belarus without control. Evaluation of possibility of volume reduction for these residuals will help to address the feasible disposal options and cost-effectiveness of the treatment technologies proposed both for radioactive domestic hearth ash and ash residuals after combustion of contaminated woody waste at the biomass fired industrial facilities. In order to prove feasibility of contaminated ash volume reduction the relevant treatment methods were tested during the bench-scale experiments. The effectiveness of acid washing technique to reduce activity levels of low-level radioactively contaminated ash to levels suitable for free-release was studied. The analysis of chemical and radiological composition of initial ash and resulting residuals was performed to evaluate potential treatment options. The actual hearth ash collected from domestic ovens and stoves in contaminated regions was used in this study. The possibility of use of encapsulated ash in form of solidified thermoplastic blocks as a construction material was also investigated. It was shown that the effective extraction of radionuclides from ash may be achieved and utilization of by-products may be feasible but the both options need the specific economic evaluation and change of existing infrastructure and regulations in a field of waste management.
INTRODUCTION
The biomass fired power plant may provide the most cost-effective and feasible means for remediation of forest contaminated as a result of the Chernobyl Accident. When applied in industrial scale, radioactive biomass conversion will lead to sufficient volume of radioactive ash. A boiler of 50 MW may produce 2.5-6.0 thousand tons of ash per year. Contaminated bottom and, particularly fly ash will have activity level of about a hundred of that for wood, and therefore may reach the parameters of LLW. Although bioenergy from contaminated wood resources is quite attractive option of remediation concept, the problem of management of a great volume of resulting ash seams to be rather serious disadvantage of this idea. There is another problem related to significant volume of radioactive hearth ash presently generated in domestic ovens by residents living in contaminated regions of the Republic of Belarus. More than 200,000 tones of radioactive hearth ash have been already collected by rural population and dispersed in back yard as a fertilizer. Thousands of tons of very contaminated ash (>50,000 Bq/kg) from municipal boilers are temporary stored waiting for appropriate treatment and immobilization.
Brookhaven National Laboratory, USA in co-operation with Institute of Power Engineering Problems, Belarus performs study of the hearth ash treatment technology based on use of the thermoplastic processes to immobilize radioactive ash. The preliminary results of this study are as follows [1]:
The next stage of this study is to evaluate the potential approach for management of contaminated ash including reduction of volume of matter to be disposed off by means of extraction of source term from ash and further utilization of the resulting residuals. Among other alternatives there is a possibility to utilize encapsulated ash of very low activity as a marketing good. These two options are discussed in the present paper.
CHARACTERISTICS OF THE HEARTH ASH
The hearth ash was sampled from domestic stoves in different regions of Belarus characterized by different levels of contamination and types of radionuclide deposits. As it was shown in our previous study [1] the content of 137Cs in hearth ash from fuel wood depends linearly on level of soil contamination in nearest forest. It was also shown that due to peculiarities of combustion process a domestic hearth furnace generates ash with higher radionuclide concentration than a domestic grade stove does. In our previous study we also concluded that ash collected has relatively high migration ability of the radionuclides: 30-40% of 137Cs and 16-40% of 90Sr have soluble and/or exchangeable forms. The difference was discovered between radionuclides behaviour in ash collected in Gomel and Mogilev Provinces. This is explained by different forms of deposit of radioactivity, released from the exploded 4th Unit of the Chernobyl NPP, presented in those regions: mostly wet deposit was in Mogilev Province and relatively dry deposit occurred in Gomel Province located close to Chernobyl Zone. For further analysis all ash samples were integrated. To provide the optimal routes of radionuclides' extraction from the hearth ash the study of ash elemental and phase composition was carried out.
About 5 kg of contaminated ash sampled in a number of domestic stoves located in the Chernobyl regions was sieved through screens with mesh diameters of 1 mm and 3 mm. The specific activity of 90Sr and 137Cs in the fractions of ash were determined as well as mass quota of each fraction was measured (Table I). The fractions of ash with particle diameters more than 1mm were collected for further experiments.
Germanium-lithium detector and Canberra-Paccard multi-channel analyzer was employed for detection of 137Cs. Detection of 90Sr was performed by emission of his daughter radionuclide 90Y using b -spectrometer with p-terphenil scintillator (80x16mm) in energy range 1.0-2.2 MeV. Apart from the instrumental method of determination of 90Sr content, a radio-chemical procedure was carried out. This method includes: (i) oxalate precipitation and calcination; (ii) consecutive precipitation and following dissolution of carbonates, Fe(III) and Y(III) hydroxides; (iii) final precipitation of yttrium oxalate. The concentration of hydrogen-ions (pH) in aqueous extract was determined. In these experiments 20g of ash were mixed with 200 ml of water.
Table I. Some Physico-Chemical Parameters of Different Granulometric Fractions
of the Ash
|
Parameter |
Value |
||
|
Granulometric class of particles |
Diameter<1mm |
3mm>Diameter>1mm |
Diameter>3mm |
|
Mass quota, % |
74 |
13 |
13 |
|
Cs-137 specific activity, kBq/kg |
32.5 |
25.0 |
20.0 |
|
Sr-90 specific activity, kBq/kg |
3,7 |
2,2 |
1.8 |
|
pH of aqueous extract |
11.7± 0.6 |
11.4± 0.6 |
11.5± 0.6 |
Elemental composition of the ash is given in Table II. These results are obtained using atomic-emission spectrometer. The procedure of probe preparation includes its digestion by mixture of concentrated HNO3 and HCl with addition of H2O2. Determination of Si content was performed using digestion of probe by HF.
Table II. Elemental Composition (Wt%) of Two Fractions of the Ash According
to the Data of Inductively Coupled Plasma-Atomic Emission Spectroscopy
|
Element |
Fe |
Si |
Ti |
Ca |
Mg |
Al |
K |
Na |
P |
|
Particle diameter<1mm |
0.60 |
11.3 |
0.018 |
16.0 |
1.94 |
0.55 |
5.08 |
0.56 |
1.24 |
|
Particle diameter>1mm |
1.5 |
12.7 |
0.043 |
10.4 |
1.58 |
0.70 |
4.91 |
0.66 |
1.23 |
The advantage of radionuclide Roentgen-fluorescent analysis is the possibility of analysis of solid phase without digestion. Two radionuclides were used as sources of electromagnetic radiation. Approximate evaluation of concentrations of some elements is conducted (Table III).
Table III. Elemental Composition (Wt%) of Two Fractions of the Ash According
to the Data of Radionuclide Roentgen-Fluorescent Analysis
|
Radioactive source |
109 Cd |
241 Am |
||||||
|
Element |
K + Ca |
Fe |
Zn |
Sr |
Mn |
Rb |
Ba |
|
|
Particle diameter < 1mm |
16 |
0.5 |
0.37 |
0.37 |
0.2 |
0.07 |
4 |
|
|
Particle diameter > 1mm |
10 |
1.4 |
0.40 |
0.25 |
|
0.06 |
3 |
|
Qualitative Roentgen-phase analysis of the ash fraction of particle size < 1mm has been performed by method of powder diffraction. According to the results obtained the ash contains following phases: CaCO3 - calcite (dominates), CaCO3 -vaterite, CaO. The existence of Ca, Al2O3, SiO2, as well as CaC2, CaAl2, CaP, and Ca3P2 may be supposed. The interplanar distances of these compounds coincide with ones of above-mentioned main phases. There are also three diffraction lines corresponding to interplanar distances 1.48; 2.67; and 3.471Å. According to the ASTM card catalogue these diffraction lines relate to CaP2O6 and CaFe3O5.
Ion chromatographic analysis was also used for determination of some elements in ash. The digestion of ash was performed by boiling in 6 mol HNO3/litre and repeated evaporation and dissolution of residue in distilled water. Concentration of Cl- ion of about 10-4% in larger fraction was found.
KINETIC OF LEACHING OF RADIONUCLIDES FROM HEARTH ASH
The knowledge of the leaching behaviour of radionuclides and heavy metals presented in ash is substantial issue for making a decision concerning arrangement of disposal options. This is also important from the environmental point of view, particularly for landfill and also for hazardous metal detoxification or for metal recovery. The objective of our study is to use this parameter to evaluate possible technology allowing the extraction of the main portion of radionuclides from hearth ash, their further concentration, encapsulation and disposal. The remainders are supposed to be non-radioactive and could be either disposed off or utilized without any limitation.
The composition and leaching properties of ash from waste wood combustion in wood industry are described in the paper [2]. The major elements in the ash are Ca, K, Na, Mg, and Fe, and trace elements As, B, Cl, Cr, F, and Zn. The pH, electrical conductivity, and contents of As, Cr, Cu, F, Zn, and Cl of the leachate are given for 19 ash samples. The pH was too high, but the heavy metal content of the leachate was not a problem with regard to above-ground landfill.
The authors of paper [3] believe that the elements likely to be leached are those that occur principally as surface deposited. The elements with low boiling points (As, Br, Cd, Ce, Hg) demonstrate very high extractability. And elements with high boiling points (Al, Fe, rare earth elements) show low extractability.
Interesting results illustrating the migration ability of metal ions in wood ash leachate were got in work [4]. The study of wood ash from lumber mills as landfill cover material in Tuolumne County, California, indicated that actual landfill leachate had less capability of mobilizing trace metals from the waste products than distilled water does. The attenuation of the migration of metals from wood ash by the landfill leachate is due to absorption and adsorption of the metal ions by fine organic matter in leachate. This organic matter is removed from the leachate through filtration by the liner in the landfill.
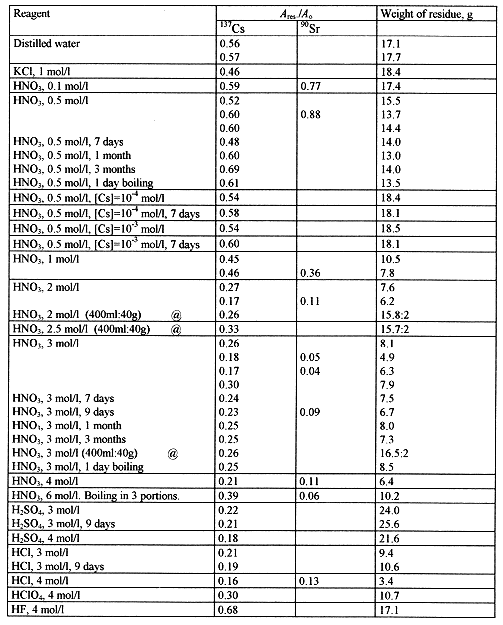
During our tests two fractions of the ash were investigated: fraction with particle diameter less than 1mm, and fraction with particle diameter more than 1mm. Twenty grams of the ash were contacted with 200ml of corresponding extragent at periodical agitation during 24 hours (if other duration is not stated). Residue undissolved was filtered through the paper filter and dried until constant weight at 100-150C. Then specific activity of 137Cs and 90Sr was measured by the methods discribed above. The ratio between specific activity of residue and that of initial ash (Ares/Ao) was used to evaluate the efficacy of radionuclide extraction from the ash. The results obtained are presented in Tables IV and V.
The dependence of extraction degree of 137Cs and 90Sr radionuclides was investigated as a function of the following parameters: acidity (distilled water; 0.1, 0.5, 1.0, 2.0, 3.0, 4.0, and 6.0 mol/l of HNO3), nature of extragent (KCl, HNO3, HCl, H2SO4), duration of extraction, temperature.
The efficacy of 137Cs extraction is increased with increasing of acidity of reagent used. The magnitude of 137Cs extraction (R) changed from 0.5-0.6 for distilled water to 0.15-0.25 for 2-4 mol/l of acid. It may be supposed that extraction of 137Cs is due to dissolving of the ash rather than ion exchange desorption. The optimal acidity of the extragents seems to be 2-3 g-equiv/l. There is not a great difference in behaviour for different fractions of ash which were investigated. The 90Sr seems to be not as strong fixed in the ash as 137Cs. The efficacy of 90Sr removal from the ash is changed from R=0.5 for distilled water to R=0.05 for 4 mol/l HNO3. It is worth to note the higher desorption ability of 90Sr than that of 137Cs from the soils contaminated after Chernobyl accident. This is accounted for by selective adsorption of 137Cs and its diffusion in mineral particles with ultimate fixation in the interlayer sites of solid particles. So the better ability of 90Sr to be extracted from the ash may be caused by the same reasons.
Table IV. The Efficacy of Radionuclide Extraction From the Ash By Different
Reagents. Fraction of the Ash With Particle Diameter More Than 1mm
|
Reagent |
Ares/Ao |
Weight of residue, g |
|
|
|
137 Cs |
90 Sr |
|
|
Distilled water |
0.48 |
|
|
|
KCl, 1 mol/l |
0.52 |
|
18.3 |
|
HNO3, 0.1 mol/l |
0.56 |
0.55 |
17.4 |
|
HNO3, 4 mol/l |
0.28 |
0.14 |
9.7 |
|
HNO3, 6 mol/l. Boiling in 3 portions |
0.25 |
|
11.2 |
Table V. The Efficacy of Radionuclide Extraction From the Ash by Different Reagents. Fraction of the Ash With Particle Diameter Less Than 1mm.

The efficacy of 137Cs extraction increases with increasing of acidity of reagent used. It may be supposed that extraction of 137Cs is due to dissolving the ash rather than ion exchange desorption. Indeed increase of the salt concentration does not promote leaching of 137Cs. Moreover we can see that increase of the 137Cs extraction is accompanied by decrease of mass of residue. This is true for all extragents except HClO4 and H2SO4. Their unusual behaviour is due to formation of the hardly soluble compounds i.e. KClO4 and CaSO4. In so far as the chemical properties of Cs+ are closer to those of K+ rather than Ca2+. The extraction of 137Cs by HClO4 is lower in comparison with H2SO4 and other acids. This is due to the more strong fixation of 137Cs in the KClO4 deposit.
The optimal acidity of the extragents (HNO3, HCl) seems to be 2-3 mol/l. There is no great difference in behavior for fractions of the ash with different particle diameter.
The 90Sr seems to be not as strong fixed in the ash as 137Cs. It is worth to note that in the case of soils contaminated after Chernobyl accident 90Sr shows higher desorption ability than 137Cs. This is accounted for by selective adsorption of 137Cs and its further diffusion in mineral particles of the soil with ultimate fixation in the interlayer sites of the solid particles. The worse ability of 137Cs for extraction from the ash in comparison with that of 90Sr may be caused by the same reason.
According to the results obtained temperature of the solution and duration of the leaching does not influence the efficacy of 137Cs removal. It may be assumed that extractable part of the radionuclide is leaching from the ash in first hours of its contact.
The same ratio of the ash extragent but twice as large amount of the ash and acid were used in the experiments denoted in Table V by @. The extraction is not very high. It may point to the importance of the hydrodynamic factor (intensity of mixing, etc.).
TREATMENT OF THE EXTRAGENTS
The chemical composition of the extracting solutions investigated is simple enough. There are either water or aqueous solutions of potassium chloride, or solutions of nitric acid with small additions of stable caesium chloride. Since the ash is composed predominantly from carbonate and oxide of calcium which are soluble in aqueous solution of such acids as nitric or hydrochloric, the aqueous phase after extraction should contain calcium cation at concentration not higher than 1 mol/l (at condition that not more than 10 g of CaO is added to 200 ml of extracting solution).
The sorption behaviour of 137Cs and 90Sr radionuclides with regard to some sorbents produced in Belarus in solution containing 0.5 mol/l of NaNO3 and 0.1 mol/l of HNO3 or NH4NO3 was studied. The following natural sorbents were investigated: cellulose phosphates, clynoptilolit and sorbents on the basis of peat. The data obtained are gathered in the Table VI from which it follows that sorption capacity of the above sorbents with regard to caesium and strontium radionuclides is very high (Kd = 103 - 104).
Table VI. Sorption of 137Cs and 90Sr
|
Sorbent |
137 Cs |
90 Sr |
||||
|
|
pH |
Kd´ 10-3 |
% of sorption |
pH |
Kd´ 10-2 |
% of sorption |
|
Polyphosphate of cellulose modified ferrocyanide nikel Ni2+ |
1,0 |
6,3± 1,8 |
97,8 |
- |
- |
- |
|
Polyphosphate of cellulose, |
- |
- |
- |
1,3 |
5,4± 1,2 |
68,2 |
|
Polyphosphate of cellulose, Mo-form |
- |
- |
- |
1,1 |
1,7± 1,2 |
69,2 |
|
Clinoptylolite |
1,3 |
9,6± 1,3 |
91,2 |
1,5 |
2,1± 1,0 |
56,0 |
|
Peat granulated, H+-form |
1,0 |
0 |
0 |
1,0 |
1,5± 1,1 |
27,3 |
|
Peat granulated (natural) |
1,6 |
0,8± 0,2 |
17,8 |
1,0 |
0,2± 0,1 |
5,0 |
UTILIZATION OF VERY LOW RADIOACTIVE ASH
In some cases after treatment of radioactive ash the resulting residues and by-products may fit the existing regulations in terms of activity level, and therefore can be utilized as valuable materials in different fields of industry and agriculture.
Ash itself if non radioactive has a great number of applications [5], e.g., fly ash from wood combustion is habitually used as fertilizer. On the other hand, in Belarus one can define very few commercial opportunities for wood ash used in industry due to absence of specific infrastructure and relevant technologies of wood ash residues utilization. For the time being the secondary (recycled) materials have not a good market yet in Belarus because of the fact that for a long time there was no lack of raw materials in Belarus which was well supplied from rich Russian resources.
In Belarus ash and slag wastes of fossil fuel-fired power plants and heat supply stations are used for cement production as fine aggregate for concrete and grout mixtures preparation, for asphalt-concrete in accordance with appropriate technical conditions for type of production. Total annual production of cement in the country exceeds 1.4 million tons. Total ash and slag waste annually utilized in construction achieves about 80 thousand tons.
Dry ash coming up at the fossil fuel-fired power plants as a result of burning powdered coals is used as a constituent for production of heavy, light, porous concrete and grouts for saving cement, and for improvement of technological properties of concrete and grouts mixtures, as well as quality properties of concrete and grouts. Annual output of ash and slag materials from power production industries constitutes about 4 million tons. Their utilization scale in the whole industry does not exceed 8%.
Ash matter is subdivided into 4 types depending on quality:
Essential factor restraining intensive application of ash and slag wastes in civil construction is radioactivity. Specific activity of natural radionuclides in ash used for construction of houses should not exceed: for 226Ra -1x10-8 Ci/kg, for 223Th- 7x10-9 Ci/kg, for 40K - 1.3x10-7 Ci/kg.
For the time being fire-wood ash is not used at industrial scale. Most of the ash residues produced at wood-fired boilers is used in agriculture as a fertilizer. Encapsulated ash in form of solidified thermoplastic blocks can be used as a construction material in many fields, e.g., roadway patching, paving tiles, roofing materials, corrosion resistant tanks, pads, pallets, containers and barriers. On the other hand, in Belarus the specific infrastructure (i.e. regulations, technologies) for application of such the goods has not been developed yet. Another restraint is an activity level which is defined for construction materials in civil and industrial fields to be no more than 3.75x10-8 Ci/kg for 137Cs.
CONCLUSION
The mean specific activities of 137Cs in the ash residuals are 2.7.10-7 Ci/kg, 8.10-7 Ci/kg and 1.7.10-6 Ci/kg for the zones 1-5 Ci/km2, 5-15 Ci/km2 and 15-40 Ci/km2 correspondingly. It was shown that approximately 50% of 137Cs and 80% of 90Sr can be removed by simple solutions, e.g., HNO3. Since mean activity of hearth ash in the maximally contaminated zone is 1.7.10-6 Ci/kg a decrease of the ash activity even by 50% will transfer the ash from the category of "radioactive waste" into the category "non radioactive waste" with activity less than 0.3 10-6 Ci/kg. The content of radionuclides in the desorption solutions can be decreased below the intervention limits for water (according to the regulations) by application of sorbents.
However, in our opinion, the further study is necessary in order to achieve more deep purification of ash from 137Cs and 90Sr radionuclides (up to specific activity of 10-8 Ci/kg, for example). This might be achieved by introduction of some cations (NH4+, K+, Ca2+, and others) and complexing anions (C2O42-, CO32-, Fe(CN)63-,4-, and others) in composition of extracting solution. The solution derived could be immobilized without pretreatment by cementation with consequent burying the cemented waste. The purified ash could be used as a mineral furtilizer or raw material for construction industries without any limitations.
ACKNOWLEDGEMENT
This work is supported by the U.S. Department of Energy under the Initiatives for Proliferation Prevention Program, and the Government of the Republic of Belarus. The authors acknowledge their sincere gratitude to Nikoly Prokshin and other specialists of the laboratory led by Prof. Yu. Davydov for their valuable assistance during the experiments.
REFERENCES