CHARACTERIZATION OF THE RADIOACTIVE GLASS
CURRENTLY BEING PRODUCED BY THE DEFENSE WASTE
PROCESSING FACILITY AT THE SAVANNAH RIVER SITE
N. E. Bibler, J. W. Ray, T. L. Fellinger, O. B. Hodoh, R. S. Beck, and O. G. Lien
Westinghouse Savannah River Co.
Savannah River Site
Aiken, SC, USA 29808
ABSTRACT
This paper presents results of the characterization of the radioactive glass being produced from the vitrification of the high level waste radioactive sludge in waste tank 51 at the Savannah River Site (SRS). The sludge is being immobilized into a borosilicate glass in the Defense Waste Processing Facility (DWPF) at SRS. In the DWPF the sludge is mixed with appropriate glass formers, fed to a melter at 1150° C, melted, and poured into stainless steel cylindrical canisters for permanent disposal in a geologic repository. Currently ~300 canisters have been filled - each containing ~4000 pounds of glass. Glass samples were obtained from the pour stream while Canisters 50 and 61 were being filled. These samples were sent to Savannah River Technology Center (SRTC) where they were characterized. Characterization includes determining the major nonradioactive and radioactive composition of the glass, its durability in a standard ASTM 1285 leach test, and its microstructure. Results of this characterization are presented in this paper. Results are also presented for the predicted composition of the glass from analyses at the DWPF analytical laboratory. These analyses are based on samples of the slurry feed from the melter feed tank batches that produced the glass in Canisters 50 and 61 were being filled. The DWPF and SRTC results were in excellent agreement for all the major elements including Cs-137, Sr-90, and uranium. The composition results also showed that the glass in both Canisters 50 and 61 had the same composition indicating that the composition of the feed to the melter was constant. This was further confirmed by measuring the radioactive composition of additional slurry samples of melter feed tank batches. At SRTC other radionuclides such as Tc-99, Sm-151 and the Pu and U isotopics were measured and results presented in this paper. The uranium isotopics indicated that the uranium in Tank 51 was slightly depleted in U-235. Results of the durability test at SRTC indicated that the glass was more durable the Environmental Assessment glass for the DWPF. This is one of the criteria for acceptance of the glass at a Federal geologic repository. Lastly, scanning electron microscopy examination of the glass indicated that it was amorphous and contained no crystals.
INTRODUCTION
The Defense Waste Processing Facility (DWPF) at Savannah River Site (SRS) has been immobilizing SRS's high level radioactive waste (HLW) into a borosilicate glass for approximately two years. Thus far, ~300 stainless steel (SS) canisters each containing ~3900 lbs (~1800 kg) of glass have been filled. These canisters are cylinders two feet (0.6m) in diameter and ten feet (3m) tall. The current campaign in the DWPF is the immobilization of the caustic radioactive sludge from SRS Tank 51. After proper pretreatment of the sludge in the DWPF, the sludge is pumped to an evaporator tank, glass formers are added and the slurry thoroughly mixed. After sampling and confirming that the composition of the mixture meets process control requirements, it is pumped to the melter feedtank (MFT) to make a batch of feed for the melter. The slurry is then sampled again and then sent to a melter at 1150° C. The MFT is a 11,000 gallon (4.2E04 liters) tank and contains sufficient feed for eight canisters of glass. During the filling of selected canisters, molten glass samples were taken from the pour stream using a specially designed sampler in the top of these canisters. These samples were sent to the Savannah River Technology Center (SRTC) where they were characterized. This characterization included measuring the nonradioactive and radioactive composition of the glass, measuring its durability in an ASTM standard leach test, and examining its microstructure. This characterization is part of the activities at SRS to demonstrate compliance with the Waste Acceptance Product Specifications (WAPS)1 for acceptance of the glass for permanent disposal in a geologic repository. This paper presents results of this characterization for glass in two canisters - Canisters 50 and 61. This paper also compares the compositional results for the pour stream samples with results from the process control analytical laboratory in the DWPF for samples taken from the MFT batches that produced the glass in Canisters 50 and 61. These were MFT batches 25 and 27, respectively. Purpose of the MFT samples is to confirm that each MFT batch has the proper composition for acceptance at a geologic repository. 1
PROCEDURES
Obtaining the DWPF Pour Stream Samples
Each canister in the DWPF is fitted with a throat protector while it is being filled with molten glass in the DWPF. Purpose of the protector is to ensure that if molten glass wicks from the pour stream it does not solidify on the throat of the canister itself and interfere with the welding of the permanent SS plug into the throat. When the pour stream is to be sampled, a special throat protector is used. This protector contains a small SS cup that can be inserted remotely into the pour stream to collect the sample while the canister is being filled. After the sample cup is filled, it is remotely retracted while the remainder of the canister is filled. After the canister is filled and removed from under the melter, the throat protector/sampler is removed from the top of the canister and the solidified glass physically dislodged from the cup. The glass sample is then shipped to SRTC in a shielded cask. Nominally 40 grams of glass are obtained in the sample sent to SRTC.
Glass Sample Preparation at SRTC for Characterization
At SRTC the glass is removed from the shielded cask and placed in the shielded cells. The glass is examined visually for variations or any striking features. It is then weighed (nominally 40 grams of glass are received) and placed in a specially labeled SS container for storage. This container also serves as an archival container for the glass not used in the characterization should any characterization need to repeated in future years. For characterization a portion of the of the sample is selected.
Obtaining Melter Feedtank Slurry Samples at the DWPF
For each MFT batch, slurry samples are taken by a pneumatic sampling system. Several ~25 mL samples are remotely transferred to a shielded cell in the DWPF analytical facility. Here the samples are combined, dried, and vitrified at 1150° C. The final glass is then crushed, dissolved and analyzed for the major nonradioactive and radioactive elements (excluding oxygen).
Glass Dissolution Methods
Prior to dissolution at the DWPF and SRTC, the glass samples were crushed and ground to enhance dissolution. Weighed amounts of the crushed samples were dissolved remotely by two different methods to ensure that all the elements were dissolved. The two methods were a sodium peroxide fusion at 650° C followed by a HCl dissolution and an acid dissolution in sealed vessels at 115° C using a combination of HF and HNO3 acids and H3BO3 to complex excess fluoride. The dissolved samples were then diluted to levels that could be safely removed from the shielded cells for analyses. Concurrent with each of these dissolutions, a standard glass was dissolved to confirm that the dissolutions were complete and the resulting analyses accurate. In all cases four aliquots of crushed glass were dissolved.
Analytical Methods
These analytical methods were used both at the DWPF laboratory to analyze the solutions of the vitrified slurry samples from the MFT and at SRTC to analyze the solutions of the dissolved pour stream samples. The major nonradioactive elements in the glasses were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES). Radioactive counting techniques were used to analyze for Cs-137 (gamma counting),
Sr-90 (beta counting), and Pu-238 (alpha counting). Except for uranium which is determined by ICP-AES at the DWPF, these are the only radionuclides analyzed in the DWPF laboratory. Analysis for these is for waste acceptance1 purposes as well as for process diagnostics. At SRTC many more radionuclides are determined in the pour stream samples to more fully characterize the glass. Combined with the counting techniques, inductively coupled plasma mass spectrometry (ICP-MS) is used. This technique was used at SRTC to analyze for uranium. ICP-MS is also especially suited for analysis of the trace fission products and actinides in the solutions. Results of applying this technique in support of the DWPF startup have been presented elsewhere.2
Standard ASTM 1285 Leach Test Procedure
At SRTC the durabilities of the two glasses obtained from the pour stream were measured using the ASTM 1285 standard nuclear waste glass leach test.3 This test is commonly referred to as the Product Consistency Test (PCT). Purpose of the test was to confirm that the DWPF was indeed producing a glass that had a durability specified by the WAPS for repository acceptance.1 This system is based on measurements of the composition of the slurries being vitrified. The ASTM 1285 test is a crushed glass (100 to 200 mesh) leach tests at 90° C for 7 days using deionized water in sealed stainless steel vessels. The test was performed in quadruplicate for each glass. Duplicate blanks and triplicate samples of a standard glass and the Environmental Assessment (EA) glass4 are also tested with the samples. Purpose of the blanks is to measure impurities than may be in the water and or leached from the vessels. The standard glass is to determine that all the parameters in the test such as sieving were carefully controlled. The EA glass is necessary for the comparison prescribed by the waste acceptance criterion for DWPF glass which states that the normalized releases for B, Li, and Na for the glass produced must be at least two standard deviations less than the respective releases for the EA glass.1 In the test, ten milliliters of deionized water are used for each gram of glass. Nominally 1.7 grams of glass were used in each stainless steel vessel. After 7 days at 90° C, the containers are removed from the oven, allowed to cool, weighed to determine water loss, and then opened. Due to the radioactivity of the glass this portion of the test was performed remotely in a shielded cell using manipulators. The leachate from each steel container is decanted into a clean vessel. The radioactivity of the leachate is low enough so it can be safely transported to a radiochemical hood where the analyses are completed. The pH of the leachate is measured and then it is filtered through a 0.45 micron filter and acidified to 1 volume % HNO3. Concentrations of B, Li, and Na are then determined using ICP-AES. These are the best elements to measure to indicate the durability of the glass for their concentrations in the final leachate are not affected by solubility constraints.
RESULTS AND DISCUSSION
Nonradioactive Composition
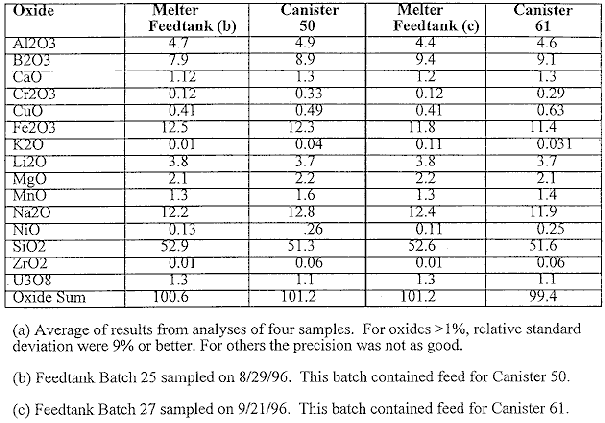
Table I compares the oxide compositions of the pour stream glasses determined at SRTC with the compositions determined at the DWPF for MFT samples of the feed to the melter when glass was being poured into Canisters 50 and 61. The results are averages from analyses of four aliquots from the respective glass samples. For the major oxides (>1wt.%) the percent relative standard deviations ( %RSD) were 9% or better. For oxides of lower concentrations the precision was not as good. . The sums of the individual oxides are also shown in the table. If all the results were completely accurate, these sums would be exactly 100.0%. They are all very close to this. The results in Table I indicate that there is the excellent agreement between the two analytical laboratories. This confirms that the composition of the glass being poured can be accurately predicted from analysis of slurry samples taken from the MFT. The results also indicate the compositions for Canister 50 and 61 are essentially identical even though Canister 61 was filled approximately one month or 11 canisters later. This is a confirmation that the composition of the feed stream being fed to the DWPF melter is not changing.
Radioactive Composition
Table II compares the concentrations (Ci/kg glass) of the major radionuclides (except uranium) measured at SRTC with those measured at the DWPF in the slurry samples taken from the MFT for the batches that contained feed for 50 and 61. The results are averages from analyses of four glass aliquots for the respective samples. The results agree within the precision of the analyses (10-15%) except for those for Pu-238. These results differ by ~30%. The DWPF uses a Pu extraction technique follow by alpha counting while SRTC uses a gross alpha measurement corrected by all the other alpha emitters present in the glass. These are primarily Cm-244 and Am-241. Efforts are now being made to reconcile this difference; however the results in Table II do indicate that the radioactive composition of the feed to the melter is essentially constant. This is also shown by the data in Figure 1 where the measured concentrations of Cs-137, Pu-238 and total beta in becquerels per gram of glass are plotted as a function of MFT batch number out to batch 35. These batches were processed over a period of nine months in 1996 and 1997. These results are routinely obtained in the DWPF waste acceptance. Prior to batch 25 the radioactivity was diluted due to the nonradioactive simulated sludge heels remaining in the melter and DWPF process vessels from the series on nonradioactive qualification runs performed in the DWPF. These runs were performed prior to receiving radioactive sludge from SRS Tank 51. For batches 25 and greater the composition of the feed is not changing. Since each canister contains nominally 40E02 lbs. (1.8E03 kg) of glass, each canister being poured contains nominally 44 Ci of Cs-137, 360 Ci of Sr-90, and 36 Ci of Pu-238.
Table III presents the concentrations of addition radionuclides measured at SRTC in the two pour stream samples. Except for Am-241, which was determined by gamma counting, all the concentrations in Table III were measured by ICP-MS. Zr-93, Tc-99, and Sm-151 are fission products of U-235 while all the others except uranium are neutron activation products. These radionuclides are examples of those that have to be reported for waste acceptance of the glass for disposal in a federal geologic repository.1 Most of the concentrations in canisters 50 and 61 are equal within experimental error (nominally 10 to 20%) especially for those radionuclides with concentrations >0.0004 wt.%. The isotopic composition of the uranium indicates that it is slightly depleted. The fractions of U-235 and U-238 in the uranium are 0.50 and 99.50%, respectively. Natural uranium is 0.72% U-235. This indicates that this uranium was probably used as a target in the SRS reactors to produce Pu-239 rather than as a reactor fuel.
ASTM 1285 (PCT) Durability Results
At SRTC, quadruplicate samples of the ground glass were subjected to the PCT along with the appropriate blanks, standard glass and EA glass as prescribed by the procedure. The results for the standard and blanks indicated that the test was acceptable. Average normalized releases measured for the glasses from the pour stream to Canisters 50 and 61 are presented in Table IV. The normalized releases were calculated from the following equation and the composition in Table I.
NRi = Ci/(Fi.1000)
where:
NRi = the normalized release based on element i
Ci = the concentration (ppm) of i in the leachate measured by ICP-AES
Fi = the weight fraction of i in the glass.
Values for the normalized releases based on the specific elements in the glass are a measure of the concentration of glass (grams/liter) dissolved in the PCT leachate based on that specific element. The standard deviations obtained in the quadruplicate tests, and the percent relative standard deviations based on B, Li, and Na, are also reported in Table IV. Measured values for the EA glass4 are 16.7 g/L for B, 9.7 for Li, and 13.3 for Na. The normalized releases reported in Table IV indicate that both glasses meet the WAPS acceptance criterion which states that the normalized releases for B, Li, and Na for the glass produced must be at least two standard deviations less than the respective releases for the EA glass.1 Both glasses are nominally 10X more durable than the EA glass.
MICROSTRUCTURAL EXAMINATION
Samples of glass from both pour stream samples were examined using a contained scanning electron microscope. The resulting micrographs showed no crystals suggesting that both samples were amorphous.
CONCLUSIONS
The results presented in this paper support the following conclusions:
REFERENCES
Table I. Comparison of Major Nonradioactive Composition (wt.%) for
Vitrified Samples from the Melter Feedtank and Melter Pour Stream
for Two DWPF Canisters (a)

Table II. Comparison of Major Radioactive Composition (Ci/kg glass) for
Vitrified Samples from the Melter Feedtank and Melter Pour Stream
for Two DWPF Canisters (a)
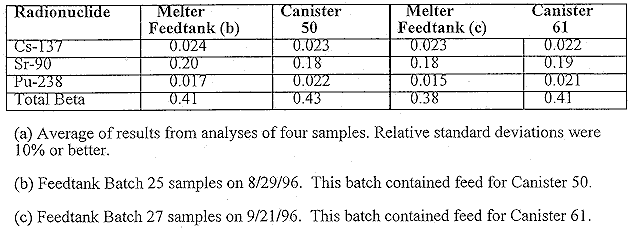
Table III. Additional Radionuclides Measured at SRTC in the Pour Stream
Samples for DWPF Canisters 50 and 61(a)
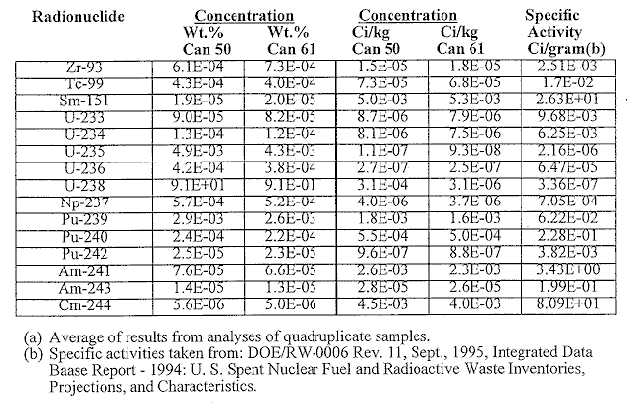
Table IV. Normalized PCT Releases (grams glass/L) for Glass in DWPF
Canisters 50 and 61(a)
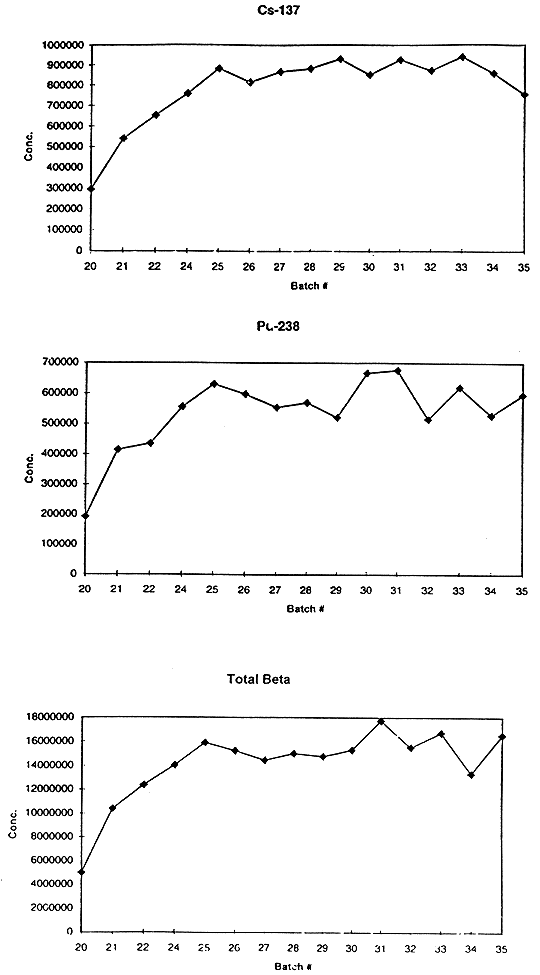
Fig. 1. Concentrations in Becquerels (radioactive disintegrations/second)per Gram of Glass of Vitrified Samples of Several Batches of DWPF Melter Feed Tank Slurries. Results are Presented for Cs-137, Pu-238, and Total Beta Activities.