NONFLAMMABLE, LOW ENVIRONMENTAL IMPACT
NONAQUEOUS CLEANING SOLVENTS
Patrick Dhooge, Suzanne Glass, and Jon Nimitz
Environmental Technology and Education Center
4500-B Hawkins St. NE
Albuquerque, NM 87109-4517
(505) 345-2707
ABSTRACT
ETEC is developing a new class of cleaning solvents, based on fluoroiodocarbons (FICs), in projects sponsored by Wright Laboratories MLBT and NASA KSC. FICs have been shown to have excellent physical properties, zero ozone-depletion potential (ODP), extremely low global warming potential (GWP), and good thermal stability. Toxicity studies have shown that the main FIC of interest in solvents is not mutagenic in the Ames test, not clastogenic in the human lymphocyte chromosome aberration test, and has relatively low inhalation toxicity. Although the cost of the FIC solvent is greater, it can be used in closed-loop systems to clean items with less solvent and decreased emissions. The FIC-based solvent can be combined with Hughes Aircraft's SPECIALÒ micro-volume closed loop cleaning system. Results showed overall good to excellent cleaning ability for Ikon Solvent P and Ikon Solvent M applied to various materials and soils, comparable and in some instances better than CFC-113. Ikon Solvent P was superior to CFC-113 in removing perfluorinated greases from all surfaces. In general, Ikon Solvent P performed better than Ikon Solvent M on typical soils. It is concluded that the use of FIC-based solvents can reduce the environmental effects of cleaning operations, and in combination with closed-loop systems can greatly reduce the amount of waste generated from critical cleaning operations.
INTRODUCTION
Many manufacturing operations, equipment uses, and decontamination operations depend critically on the final cleanliness of surfaces. These surfaces include, for example, those of mechanical and test devices, optics, electronic components, scrap equipment, D&D wastes, and the surfaces of containers.
Surfaces can become contaminated with adsorbed films, particulates, and gases. Soils may be categorized as organics, inorganics, and particulates. Organic soils (films) of concern include oils, greases, and waxes. Inorganic (ionic) contaminants include acid residues (e.g., carboxylates, sulfates, chlorides, bromides, lactates, nitrates, sulfates, fluorides), and base residues (e.g., ammonium, sodium, and potassium hydroxides plus amine and polyamine salts). Particulate matter includes airborne dusts of all types and colloidal silica from degraded deionized water. Gases and airborne materials can also contaminate surfaces.
Substrates of interest include metals, composites, ceramics, optical surfaces, and electronic components including semiconductor wafers. Metals of interest include, for example, aluminum alloys, stainless steel, titanium, copper, uranium, and plutonium.
Chlorofluorocarbon (CFC) and chlorocarbon solvents are excellent for removing a variety of dirt from surfaces. Unfortunately, CFCs and chlorocarbon solvents have deleterious effects on the ozone layer, are greenhouse gases, and have various toxic effects. Due to their ozone depletion potential (ODP), the Montreal Protocol and its amendments, as well as other environmental regulations, have dictated the phaseout of ozone-depleting solvents such as CFC-113 and 1,1,1-trichloroethane [1-4]. Additional environmental regulations have required reduced emissions of volatile organic compounds (VOCs).
Analytical methods for testing cleanliness of parts and determining organic contaminants in wastes have also relied on CFCs and chlorinated solvents [5]. Although alternatives have been recommended, they do not perform as well as the original solvents and some analyses, such as the infrared analysis of extracted hydrocarbons, cannot be performed with the substitute solvents.
CFC-113 solvent has been used for several critical applications in the nuclear weapons complex. The method for cleaning highly enriched uranium (HEU) specifies CFC-113 for washing oils and greases from uranium chips [6]. Plutonium has also been cleaned with CFC and chlorinated solvents. While much ferrous and nonferrous parts cleaning has now been converted to aqueous-based cleaning agents, aqueous-based agents cannot be used for cleaning reactive metals such as uranium and plutonium. Also, electronic components in equipment can be damaged by aqueous-based cleaning agents. CFCs have been used extensively as precision cleaning solvents by nuclear electric power facilities and in laboratory operations and analyses. In 1990, of the 140,000 ft3 of mixed waste generated in the United States, perhaps as much as 10% was CFCs, including 27% of the waste from the nuclear utility industry [7]. Solvents have been identified by EPA as one of the primary areas for waste reduction for nuclear powered electrical generating stations [8].
Several new cleaning solvents and techniques have appeared in response to the phaseout of CFC and chlorocarbon solvents. Various hydrocarbon and oxygenated hydrocarbon solvents have been introduced or their use expanded, but these compounds are flammable and do not clean as effectively. Isopropyl alcohol (IPA) can be used in many cases, but elaborate engineering is needed to minimize the flammability risk. As a result, systems designed for IPA cleaning in non-radioactive operations are expensive (on the order of $100,000). Flammability is a particularly critical safety concern in nuclear operations, causing capital and operating cost increases well in excess of those encountered in non-radioactive operations. Additionally, in IPA cleaning large volumes of IPA are often used as final rinse and dry, generating large volumes of waste. This can be quite expensive in nuclear operations, due to the cost of disposing of radioactive wastes. Hydrofluorocarbons (HFCs) have zero ODP but poor cleaning effectiveness. Supercritical carbon dioxide systems have been introduced, but they require pressurized equipment and are limited in size. Improving surfactants and equipment has expanded applications of water-based cleaning systems, but these systems tend to leave residues and are not suitable for all materials. Plasma cleaning is being developed as a solvent-less process, but it requires sophisticated equipment and is also not suitable for all materials. Some applications of alternative cleaning processes have been cost-effective. However, many alternative solvents and methods are expensive to implement and give incomplete cleaning or leave residues that compromise product quality.
The trend in solvent waste regulations is toward tighter restrictions and higher costs. As a result, the already high costs of monitoring, collecting, and disposing of wastes are likely to continue to rise. Closed-loop systems are becoming more attractive for many uses because they prevent emissions and allow virtually complete recovery of solvent, thus minimizing wastes and allowing cost-effective use of more expensive solvents.
Conventional wet-chemistry surface cleaning processes in use today (such as squirt-bottle rinsing, immersion, spraying, and flushing under a running tap) lack the precision, cleanliness, and parametric control that is so critical for high-performance processing. In addition, current processes may at times expose workers to toxicity and flammability hazards, and may generate large quantities of waste. New solvents and methods of cleaning are needed to eliminate these concerns. At this time no surface cleaning method exists that has excellent cleaning ability, high reproducibility, low environmental impact, zero flammability hazard, low toxic hazard, engineering simplicity, and low cost.
FIC SOLVENTS
ETEC is developing a new class of nationally and internationally patented solvents, fluoroiodocarbons (FICs), in projects sponsored by the US Air Force Wright Laboratories MLBT and NASA KSC. The effort for Wright Laboratories MLBT is developing new environmentally-safe nonaqueous solvents for removal of engine oils, hydraulic fluid, and perfluorinated grease in Air Force ground maintenance operations. The effort for NASA is developing nonaqueous solvents for critical cleaning operations on oxygen and other aerospace propellant systems.
Fluoroiodocarbons have many of the attractive properties of chlorinated and chlorofluoro solvents, but since they contain one or more atoms of iodine they decompose much more easily in the environment. The main FIC of interest has been shown to photolyze completely in the atmosphere within 2 days, so FIC solvents will not be a long-term atmospheric hazard. However, as long as they are not exposed to significant amounts of UV light, they are quite stable at typical storage and use temperatures.
FICs have low environmental impact. Because of their zero ODP, they are not subject to phaseout under the Montreal Protocol as amended. FICs also have extremely low global warming potential (GWP), which means that they will not be as subject to any use limitations based on GWP.
Toxicity studies have shown that the main FIC of interest in solvents is not mutagenic in the Ames test and not clastogenic in the human lymphocyte chromosome aberration test. 28-day rat subchronic inhalation (6 hrs per day) exposure tests showed no effect from the main FIC of interest at 1,000 ppm exposure, and only small effects (low weight and enlarged livers in the males) even at 10,000 ppm exposure. Beagle dog cardiac sensitization inhalation tests showed a No Observable Adverse Effects Limit (NOAEL) of 0.4% (4,000 ppm), and a Low Observable Adverse Effects Limit (LOAEL) of 0.6% (6,000 ppm).
Although the cost of the FIC solvents is greater, they can be used in closed-loop systems to clean items with less solvent and decreased emissions. The two best cleaning solvents identified in testing have been designated Ikon Solvent P and Ikon Solvent M. An application for approval of Ikon Solvent P has been submitted to the EPA's Significant New Alternatives Policy (SNAP) Program.
COMPATABILITY
FICs are highly compatible with most common materials of construction. The primary FIC solvent of interest has been tested for compatibility with a variety of common materials of construction, based on the methods described in the American Society for Testing and Materials (ASTM) Standard D471-79 (for polymers) and Method D2251 (for metals).
Polymer types tested were Buna-NÒ rubber, butyl rubber, ethylene-propylene rubber (EPR), fluorosilicone rubber, neoprene rubber, silicone rubber, PTFE TeflonÒ , polyurethane, and VitonÒ . The FIC solvent was not compatible with Buna-NÒ rubber, silicone rubber, and polyurethane. The failures were of the nature of excessive swelling and weight gain, rather than any dissolution or decomposition of the polymer.
Metals tested were aluminum alloys 2024, 5052, 6061, and 7075T6; stainless steels 303 and 416; titanium; mild steel; cast iron; magnesium; copper; brass; bronze; acid-core solder; plumbing solder; and rosin core solder. The FIC solvent had excellent compatibility with all metals tested except mild steel. The mild steel showed significant oxidation, but the oxidation could be due to air and moisture that were not rigorously excluded. Metals and alloys such as titanium, aluminum, and stainless steel are used extensively in nuclear process equipment, and FIC solvents are compatible with all of these materials. Based on the compatibility test data with other metals, we would expect FIC solvents to be compatible with uranium and plutonium, but this has yet to be tested.
FICs are compatible with other common solvents, water, adsorbents, and plastics at normal temperatures. FICs are not expected to be compatible over long storage periods with strong bases or zero valence alkali metals, and it is suggested they not be exposed to bulk fine metal powders due to possible catalytic decomposition effects. No studies have been conducted on the effects of ionizing radiation on FICs, other than the known effect of UV light in decomposing FICs.
CLEANING ABILITY
One by three inch coupons of substrate material were intentionally soiled with one of six representative soils: BraycoteÒ 601 EF (a perfluorinated grease) ; KrytoxÒ 240 AC (a perfluorinated grease); Amoco RykonÒ Grease No. 2EP (a hydrocarbon grease); Dow-Corning DC-55M (a silicone grease); Royco 782 Superclean Hydraulic Fluid MIL-H-83282 (a fire-resistant hydraulic fluid containing phosphate esters); and TribolubeÒ 16. Soiling levels were from 1.0 to 5.0 g/ft2. Each coupon was carefully weighed, then cleaned, air dried, and reweighed to determine soil removal effectiveness.
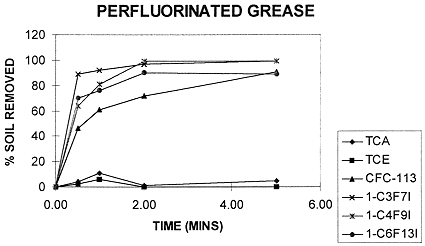
The FIC solvents were found to have excellent cleaning abilities, comparable to and in some cases better than CFC-113. In particular, they remove perfluorinated greases (i.e., KrytoxÒ 240, BraycoteÒ 601 EF, and similar) better than CFC-113, as shown in Figure 1. Figure 2 shows that a supersoil composition made up by combining the soiling agents was removed as effectively by the FIC solvent as by CFC-113.

Fig. 1. Removal of Perfluorinated Greases
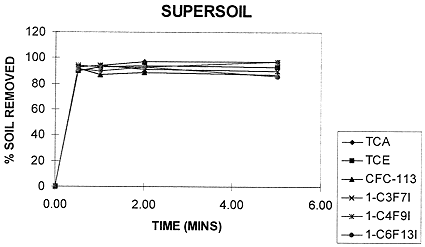
Fig. 2. Removal of "Supersoil"
The FIC solvents' cleaning abilities were tested by simple immersion cleaning, wipe cleaning, ultrasonic bath cleaning, and SPECIALÒ system cleaning (see separate section on SPECIALÒ cleaning tests below). For all methods tested, Ikon Solvent P is as effective as CFC-113 in removing greasy soils. Ikon Solvent M was found to be somewhat less effective in immersion and wipe cleaning. Ultrasonic cleaning essentially leveled the field between the two FIC-based solvents and CFC-113; within experimental error, all three solvents achieved 100% removal of BraycoteÒ 601, RykonÒ Grease No. 2, and Dow-Corning DC-55M after one minute in a BransonÒ ultrasonic bath.
Continued cleaning tests examined the ability of the FIC solvents and CFC-113 to remove a soil when the solvent was already saturated with that soil. BraycoteÒ 601, RykonÒ Grease No. 2, and Dow-Corning DC-55M were chosen as representative soils. The results of these tests were even more encouraging than the tests with clean solvents. As shown in Figure 3, Ikon Solvent P consistently removed the three soils from 316 stainless steel significantly faster than CFC-113, indicating superior cleaning ability over extended use applications.
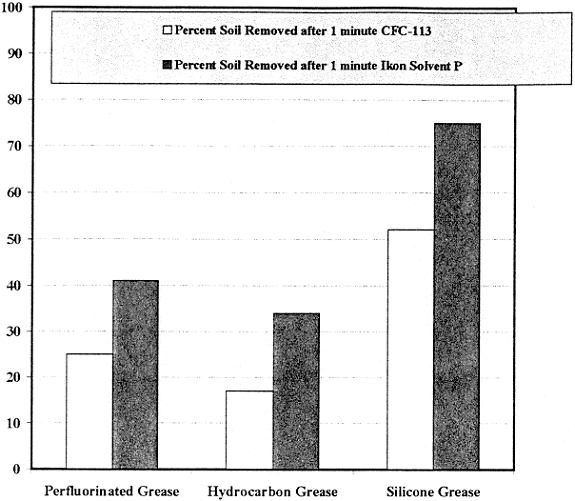
Fig. 3. Cleaning Ability of Soil-Saturated FIC Solvent vs. Soil-Saturated CFC-113
THE SPECIAL
Ò CLEANING SYSTEMThe SPECIALÒ cleaning system has been developed at Hughes Aircraft Santa Barbara Research Center. The system has these distinct advantages over many other solvent cleaning systems: (1) it minimizes the volume of solvents, etchants, and deionized water required to process parts, (2) it provides a high degree of control of the surface chemistry of cleaned or etched substrate surfaces, (3) it improves the reproducibility of wet-chemistry process parameters, and (4) it captures all chemical effluents generated by the process.
The SPECIALÒ system consists of three interconnected subsystems contained in a bench-top nitrogen-purged glove-box-type enclosure. The three subsystems are: (1) a liquid reagent dispensing compartment, (2) a novel treatment cell, and (3) an effluent capturing compartment. A load-lock antechamber on the side of the glove box can be used to move a transfer vessel in and out of the glove box in air-sensitive operations, to protect the part surface and glove box interior from contamination by air. In-situ ellipsometry allows the surface to be monitored non-destructively and serves as an end-point indicator as well as a built-in surface cleanliness diagnostic. The micro-volume pulse-wait technique used is based on the well-established principle that multiple, low-volume, surface-proximity rinses are far more effective and less wasteful for cleaning than single macro-volume bulk rinses. Instead of several hundred mL of solvents being consumed per wafer, less than 5 mL are required.
SPECIAL
Ò CLEANING RESULTS WITH FIC SOLVENTSA custom-made cleaning cell was fabricated to clean Inconel, stainless steel, and TeflonÒ substrates supplied from NASA KSC. Substrate coupons were carefully weighed, then intentionally soiled with measured amounts of the same six soils used in the cleaning tests described above. Soil loading levels were from 0.25 to 1.0 g/ft2. The coupons were then cleaned and subsequently examined by weighing, photomicroscopy, and ESCA. The cleaning period was one minute for TeflonÒ coupons, and three minutes for Inconel and stainless steel coupons. The coupons were dried in dry nitrogen for one minute after cleaning.
Within the experimental error, the FIC solvent displayed cleaning effectiveness equal to that of CFC-113. All coupons cleaned by either solvent had 99.7 - 99.9% soil removal.
CONCLUSIONS
The FIC-based solvents appear to be excellent replacements for the traditional CFCs and chlorocarbons in solvent cleaning operations. Although the cost of the FIC solvents is still high, it will decrease as the production volume increases, and in many applications the cost of solvent replacement is still much less than the cost of introducing, documenting, and validating an entirely new cleaning process.
Tests in the first-generation SPECIALÒ system showed that Ikon Solvent P is highly effective for cleaning soils from test surfaces, and demonstrated the feasibility of the micro-volume closed-loop system critical cleaning of components. The micro-volume closed-loop system is a perfect compliment to the FIC solvents for critical cleaning applications or applications where solvent emissions cannot be tolerated.
Although FIC solvents have not yet been tested in specific nuclear applications, such as uranium chip cleaning, all available information indicates that they would be highly effective, non-ozone-depleting solvents to replace CFC-113 in these uses. FIC solvents should also be an effective CFC-113 replacement in cleanliness and organic contamination analytical and testing methods related to nuclear parts cleaning and waste analysis operations.
ACKNOWLEDGMENTS
The funding for this work was provided by the US Air Force Wright Laboratories MLBT and NASA Kennedy Space Center. We would like to thank Dr. Edward Snyder of Wright Laboratories and Dr. Jamie Palou of NASA KSC for their valuable technical support during the efforts. We would also like to acknowledge the contribution of Ms. Kirby Olson, who performed many of the cleaning tests.
REFERENCES