
COMPLEX REPROCESSING OF LRW WITH ISOLATION
OF SOME RADIONUCLIDES BY SORBTION
A.A. Kopyrin, A.K.Pyartman, V.A.Keskinov, M.A.Pleshkov
St-Petersburg State Institute of Technology, 198013, St-Petersburg, Russia
I.A. Sobolev, S.A. Dmitryev, S.V.Stefanovsky
NPO "Radon", Moskow, Russia
ABSTRACT
A new method of composite sorbents synthesys, which includes cations and anions sorbtion by organic materials and preparation of composite material inside sorbents pellets, is proposed. Samples of sorbents on the basis of ion-exchange resins KU-2x8(KU-23), AV-17, VP-1AP, multivalent metal salts (Zr(IV), Fe(III), Cr(III), Mn(1V) hydroxides and phosphates, potassium and transite metals(Cu, Ni, Co, Zn(11) ferricyanides) are prepared. Composite materials selectivity to radionuclides 137Cs, 60Co, 144Ce and 90Sr separation from complex water solutions was studied.
INTRODUCTION
The opportunity of allocation represents separate long - lived radioactive elements from radioactive wastes low and average level a urgent task of modern radiochemical technology. To number long - lived radioactive elements it is necessary to relate 137Cs, 90Sr, 60Co, 59Fe, 144Ce, 152Eu and others. For allocation radioactive elements methods of extraction and sorption are used more often [1-4].
Most difficult is the problem of allocation radioactive elements from water-salts solutions of complex structure, where the presence salts largely reduces both factors of distribution and factors of division of various pairs radioactive elements and accompanying impurity.
For the decision of the specified problem can be used various inorganic sorbents and extractions reagents . However specified materials have a number of lacks - low mechanical durability, bad hydrodynamical properties, solubility in water-salts solutions etc [1-4]. The specified lacks can be eliminated with use of composite materials - inorganic sorbents, brought in structure of the ionic replacement materials and polymeric matrixes and extractives, brought in to structure of the polymeric materials.
As porous carriers can be used the polymeric materials, for example porous teflon (CF-4), active coals, for example BAU or SKT-6A, ion-exchange resins. The contents of an inorganic component in the porous carrier is usual makes from 10 up to 20 mass percents.
In the present work the composite materials are synthesized and their selectivity to a number radionuclides in water solutions containing soduim nitrate concentration up to 500 g/l is investigated.
EXPERIMENTAL PART
For synthesis of composite materials in work are used active coal BAU, ion-exchange resins KU-2x8, KU-23, AV-17, BP-1AP and porous teflon CF-4. Before synthesis all materials were processed by nitric or hydrochloric acids, sodium hydroxide and water.
As an inorganic component are used hydroxides Zr(1V), Fe(111), Cr(111), Mn(1V), phosphates Zr(1V) and Fe(111) and also mixed potassium and transite metals (Cu, Ni, Co, Zn(11) ferricyanides.
In case of porous carriers, for example active coal BAU, the way of synthesis of a composite material consist in impregnation of the carrier by salt of metal with the subsequent processing by a solution hydroxide ammonium or sodium phosphate. In a case ion-exchange resins cations or anions inorganic sorbents previously exchanged with sodium cations or chlorine-ions in dynamic conditions, and then ion-exchange resins processed by other reagent in static conditions.
For example, in case of preparation composite sorbents on a basis ion-exchange resin AV-17 and ferricyanides potassium and transite metals ways of synthesis consist in the following:
4R-Cl + [Fe(CN)6]4- Û R4 [Fe(CN)6] + 4Cl- , (1)
R4 [Fe(CN)6] + 2KCl + MCl2 Û K2 M [Fe(CN)6] + 4R-Cl, (2)
where R- functional group ion-exchange resin AV-17.
For synthesis of materials on a basis ion-exchange resins and dioxide of manganese (1V) used processing ion-exchange resins by solutions of KMnO4.
The structure composite sorbents was characterized by the data of the element chemical analysis.
The general contents of an inorganic component or the contents extractives made from 10 to 20 mass percents. The inorganic component composite sorbents can be characterized by the following structural formulas: ZrO(OH)2, ZrO(HPO4), ZrO(H2PO4)2, FeO(OH), CrO(OH), MnO(OH)2, K2 M [Fe(CN)6] (M=Cu, Ni, Co, Zn(11)) or similar depending on conditions of synthesis.
For comparative study in a number of cases were prepared inorganic sorbents without the carrier of such structure, as composite materials.
The static and dynamic exchange capacities of the received composite materials (on ions sodium and chlorine- or nitrate-ions) are determined on standard techniques. As a whole they made from 0.7 up to 2.0 mmol/g of a composite material.
It was found that if the temperature is below 2000C the change of sorbents weight is dew to dehydration processes. In the temperature range 200 -use of organic material destruction and in the range 600-900o C - because of solid oxide structures formation.
In work are used radiochemistry pure radionuclides 137Cs, 60Co, 144Ce and 90Sr etc. For definition of factors of distribution are used b and g -radiometer. For definition pH of solutions used the device pH-340 and glass electrodes.
In the majority of experiences on distribution radionuclides used solutions sodium nitrate with concentration from 100 to 500 g/l and pH =1-8. In a number of experiences used solutions sodium nitrate with the additives sodium chlorine or sodium sulfate (up to 10 g/l). The specified solutions most close approach to structure of solutions containing liquid radioactive wastes of an average and low level.
RESULTS AND THEIR DISCUSSION
In Fig.1 the dependences of the logarithm of factor of distribution 137 Cs(1) for a number inorganic COPOEHTOB (ferricyanides potassium and transite metals (Zn(11), Ni(11), Co(11)) are given depending on concentration sodium nitrate in a water solution. The increase of concentration sodium nitrate results in essential decrease of factor of distribution 137 Cs(1) , that is caused by a competition of an ion sodium with an ion cesium on the equation of reaction:
K2 M [Fe(CN)6] + 2Cs+ Û Cs2 M [Fe(CN)6] + 2K+, (3)
K2 M [Fe(CN)6] + 2Na+ Û Cs2 M [Fe(CN)6] + 2K+, (4)
and dehydration inorganic sorbents with growth of concentration sodium nitrate in a water solution.

Fig 1. The dependences of the logarithm of factor of distribution 137 Cs(1) for a number inorganic sorbents (ferricyanides potassium and transite metals ) on concentration sodium nitrate in a water solution ( mol/l). Is designated : (1)- K2 Zn[Fe(CN)6],
(2)- K2 Cu [Fe(CN)6],
(3)- K2 Co[Fe(CN)6],
(4)-K2 Ni [Fe(CN)6].

Fig 2. The dependences of the logarithm of factor of distribution 137 Cs(1) for a number composite sorbents on the basis of inorganic oeHTOB (ferricyanides potassium and transite metals ) and ion-exchange resin AV-17 on concentration sodium nitrate in a water solution (mol/l). Is designated : (1)- K2 Zn[Fe(CN)6],
(2)- K2 Cu [Fe(CN)6],
(3)- K2 Co[Fe(CN)6],
(4)-K2 Ni [Fe(CN)6].
In a Figure 2 the dependences of the logarithms of factors of distribution 137 Cs(1) are given depending on concentration sodium nitrate a water solution for composite materials - ferricyanides potassium and transite metals (Zn(11), Ni(11), Co(11)) put on exchange resins AV-17 and VP - 1AP.
As follows from the data of a Fig. 2, on factors of distribution 137 Cs(1) pass through a minimum, that is caused as a competition of ion sodium with ion cesium on the equation of reaction (3) and (4), but weaker effect dehydration composite sorbents with growth of concentration sodium nitrate in water-salts solutions in comparison with inorganic sorbents.
Thus, the composite materials can in a greater degree be used for extraction 137 Cs(1) from water-salts solutions with high concentration sodium nitrate.
On a method Boide the factors diffuses on inorganic and composite sorbents in 1.0 mol/l solutions sodium nitrate were measured, which have made 10-7-10-8 sm 2 / s depending on a nature sorbents.
The static and dynamic exchange capacities composite materials on ions cesium are determined in 1.0 mol/l solutions of sodium nitrate, which have made up to 1.0 mmol/g of a materials. In the whole selectivity of composite materials in relation to ions cesium is reduced on the following number of materials:
K2 Zn[Fe(CN)6] > K2 Cu [Fe(CN)6] > K2 Co[Fe(CN)6] >
K2 Ni [Fe(CN)6].(5)
Equation (5) is kept both for inorganic and composite materials irrespective of a nature ion-exchange resins.
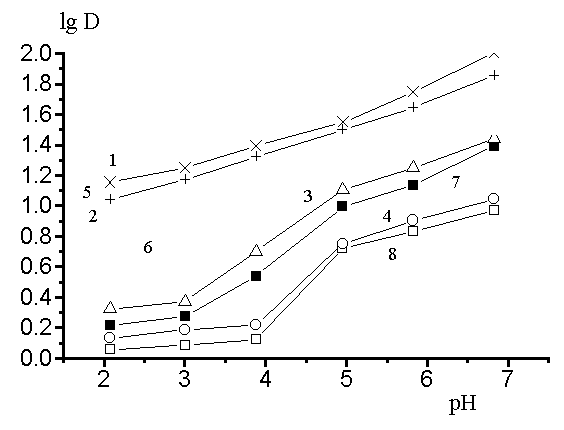
Fig 3. The dependences of the logarithms of factors of distribution radionuclides 58 Fe(111), 60 Co(11) 90 Sr(11) and 144Ce(111) on pH of solutions with use of composite materials on the basis of active coal BAU and inorganic sorbent - ZrO(H2PO4)2 (1-4) and on a basis ion-exchange resin KU-23 and ZrO(H2PO4)2 (5-8) in the 1 mol/l solution of sodium nitrate. Is designated :
1,5- 144Ce; 2,6-58 Fe; 3,7- 60 Co; 4,8- 90 Sr.
In Fig. 3 the dependences of the logarithms of factors of distribution radionuclides 58 Fe(111), 60 Co(11) 90 Sr(11) and 144Ce(111) are given depending on pH of solutions with use of composite materials on the basis of active coal BAU and inorganic sorbent - ZrO(H2PO4)2 and also on a basis ion-exchange resin KU-23 and ZrO(H2PO4)2 in the 1 mol/l solution of sodium nitrate. As follows from the data of a Fig. 3, the change pH from 2 up to 5 of a water solution sodium nitrate increases of factors of distribution radioactive elements. Optimum area is pH = 3-4, where there is a most complete extraction radioactive elements . The selectivity of composite materials is increased in line:
Ce(111) » Fe(111) > Co(11) >> Sr(11) (6)
Thus, with use of composite salts on a basis ZrO(H2PO4)2 it is possible by selection of meanings pH in water solutions sodium nitrate selectively to take a number radionuclides ( 58 Fe(111), and 144Ce(111) ) at the presence of others(60 Co(11) and 90 Sr(11)).
In work is established, that the selectivity of inorganic and composite materials in relation to radionucledes 60 Co(11), 58 Fe(111), 90 Sr(11) and 144Ce(111) is reduced in a number inorganic sorbents:
ZrO(H2PO4)2 » MnO(OH)2 > FeO(OH) » CrO(OH) .(7)
The static and dynamic capacities of the investigated materials are determined. The meanings of capacities have made 1-2 mmol/g materials on a nature of inorganic and composite materials and nature of radionuclides.
On a method Boide the factors diffuses on inorganic and composite sorbents in 1.0 mol/l solutions sodium nitrate were measured, which have made 10-7-10-9 sm 2 / s depending on a nature sorbents.
For processing liquid radioactive wastes an average and low level with the high contents of water-salts it is possible to offer the following circuit:
The offered circuit is realized on the model systems with the contents sodium nitrate in a water solution up to 300-350 g/l.
REFERENCES