CHEMICAL AND ELECTROCHEMICAL DECONTAMINATION
METHOD FOR HOT-SPOT CONTAMINATED WITH
RADIONUCLIDES ON LARGE SURFACE OF
METAL OR CONCRETE
Yuan Zhang, Xianwen Ren, Wenchang Liu,Weidong Li
China Institute for Radiation Protection
P.O.Box 120
Taiyuan, Shanxi 030006,
People's Republic of China
ABSTRACT
For large contaminated surfaces of metal or concrete, decontamination is difficult and produces a large volume of wastes (especially for concrete surfaces), even though the contamination is generally in the thin layer of the surface. A chemical decontamination apparatus we designed can decontaminate the contaminated hot-spot on the large surface of metal or concrete easily and not re-contaminate other surface areas nearby the contaminated hot-spot. The decontamination process also produces smaller volumes of secondary waste than mechanical decontamination methods.
The chemical decontamination apparatus consists of three parts. The first part is a vacuum attaching unit or mechanical attaching unit, which makes the decontaminating head of the apparatus attaching to the contaminated surface area adhere strongly and prevents the chemical decontamination solution from leaking out of the head. The second part is a chemical decontamination solution recycling-unit, which makes the decontamination solution react with the contaminated surface area continuously at a designed flow rate. The third part is a direct current supplying unit, which provides a direct current between the contaminated surface of metal and the cathode in the decontaminating head of the apparatus when decontaminating the contaminated surface of metal.
After decontamination, the contaminated solution is automatically collected into the storage tank and does not diffuse over the surface of the metal or concrete to re-contaminate the other clean surface areas nearby the hot-spot.
Key words: Decontamination, hot-spot, chemical, electrochemical.
INTRODUCTION
For decontamination of hot-spots on a large surface area of metal or concrete, the artificial scrubbing method was generally used in the past; however, this method was labor-intensive, had a high-dose burden, and was not always effective[1].
The chemical decontamination apparatus was designed to overcome those disadvantages. Different types of chemical solutions can be used on various types of surfaces according to the requirements of decontamination.
First, the apparatus is designed to contact compactly with the surface to be decontaminated. Then, the chemical solution can be limited to the space between the decontaminating head and the surface contaminated. For the decontamination of metal surfaces, the direct current can be applied between the contaminated surface (as an anode) and the decontaminating head (as a cathode) to increase the dissolving rate of the metal surface. After the decontamination process, a vacuum pump unit will pump the solution into the storage tank; thus, the contaminated surface can be decontaminated rapidly and the area nearby the hot-spot cannot be contaminated.
EXPERIMENTAL CHEMICAL DECONTAMINATION
APPARATUS AND METHOD
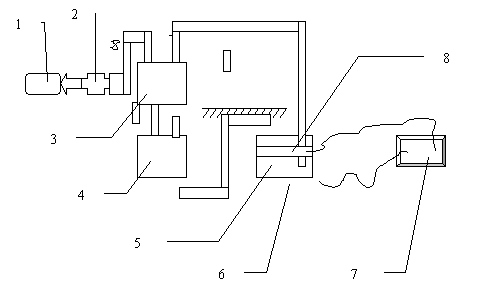
The sketch of the experimental chemical decontamination apparatus is shown in Figure 1. The functions of each part are as follows.
The vacuum pump rises to a negative pressure, and then the decontaminating head adheres to the contaminated surface compactly. The chemical decontamination solution can be limited to the space between the decontaminating head and the contaminated surface.
The filter prevents various particles that come from the decontamination process from entering into the pump and destroying it. The drier prevents the acid gas and vapor from entering into the pump.

Fig. 1. Sketch of Hot-Spot Chemical Decontamination
Notes:
| 1- Vacuum pump | 2- Filter and Drier | 3- Buffer |
| 4- Storage tan | 5- Decontaminating head | 6 - Contaminated hot-spot |
| 7- Direct current supplier | 8- Cathode |
The buffer is a negative-pressure compartment that can draw the solution from the decontaminating head into it so that the solution can run into the decontaminating head from the storage tank continuously because of the effect of the vacuum.
The storage tank is a vessel for storing the chemical decontamination solution. In the decontamination process, the solution flows out of the storage tank and into the decontaminating head, then is drawn into the buffer, and continuously flows. After or in the break of a decontamination process, the solution then comes back to the storage tank from the buffer.
The decontaminating head is made of several parts. The first part is an acid-proof, soft-sealing cushion that seals the crack between the contaminated surface and the decontaminating head. The second is a solution-cycling unit that consists of the flowing in and flowing out. The third is a cathode, powered by a a small rectifier, that applies to the decontamination of the metal surface.
The entire system is acid-proof and alkali-proof, which allows the use of several kinds of solution for decontaminating various metal and concrete surfaces.
The experiments were performed to determine the dissolving rate of metal surface and the loss of solution to the nearby area around the hot-spot.
RESULTS AND DISCUSSION
Decontamination of metal surface
The dissolving rate of stainless steel can be determined by using this apparatus. The sample is 100´ 100´ 1 mm of 1Cr18Ni9Ti. The practical area of contact with the solution is 50.24 cm2, so the weight loss can be weighed out. The decontamination solution consists of 2.0 mol/l nitric acid and a small amount of nitrate of cerium.
The results of experiments are showed in Table 1
Table 1. The Dissolving of Stainless Steel
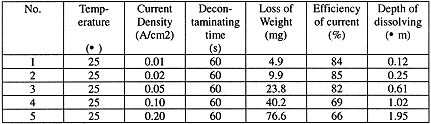
A higher dissolving rate is obtained at a higher current density, and higher dissolving depth can be achieved at higher current density or longer contacting time.
The leakage of solution to a nearby area is small because the safety controller can limit the flow of the solution when the negative pressure in the space of the decontaminating head is not maintained.
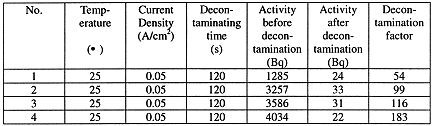
The simulated samples to be decontaminated are contaminated by sinking into the solution containing 137Cs with activity of 1.36´ 105Bq/l. The results of experiments are in Table 2.
Table 2. Decontamination of Stainless Steel Contaminated with 137Cs
Decontamination of Concrete Surface
There is no quantitative data for the decontamination of concrete surfaces; only some phenomena have been observed in the process of experiments. Decontamination of a concrete surface was achieved by eroding the surface with fluoboric acid. Further experiments will be performed to determine the dissolving rate and the treatment method of secondary waste solution.
CONCLUSIONS
This chemical decontamination apparatus can be applied to the decontamination of hot-spots contaminated with radionuclides on large surfaces of metal or concrete. It operates easily and rapidly, and reduces the dose burden of professional workers. Secondary waste is also greatly decreased.
It is concluded from our experiments that this method can be an optimistic decontamination technology, but it needs to be developed into computer-controlled equipment and fixed into a motorized vehicle, so decontamination can be quick and exact. We hope to accomplish this goal in the future if sufficient funding support is provided.
REFERENCES