![]()
DECONTAMINATION OF STAINLESS STEEL WITH CERIUM(4)
ION OXIDIZED BY ELECTROCHEMICAL METHOD
IN NITRIC ACID SOLUTION
Yuan Zhang, Xianwen Ren, Jiangquan Wang
China Institute for Radiation Protection
P.O.Box 120, Taiyuan, Shanxi 030006, People's Republic of China
ABSTRACT
The rate of stainless steel dissolving in nitric acid solution is enhanced with the addition of cerium(4). Therefore, the nitric acid solution containing cerium(4) ion can be an effective decontamination method for contaminated stainless steel. To reduce the consumption of cerium and the volume of secondary waste, the cerium(3) ion that remains after the cerium(4) ion is used to decontaminate metal should be re-oxidized into cerium(4), recycled, and reused in the decontamination method.
In our experiments, a specially designed electrolytic cell was used to oxidize the cerium(3) ion into cerium(4) ion. The structure of the electrolytic cell is simple, operates easily, and fits the requirements of decontamination operation.
The concentration of cerium(4) ion in the nitric acid solution was chosen in our experiments to dissolve the surface of contaminated stainless steel at a suitable speed. The total concentration of cerium ion also was chosen to ensure that the regeneration rate of cerium(4) ion can satisfy the consumption rate of cerium(4) ion in the decontamination process. The operation parameters were selected strictly on the basis of experiments so that the regeneration rate of cerium(4) ion could be reasonably high in proper operating conditions and not raise safety hazards that could jeopardize the safety of the operation.
This method can be applied to the decontamination of nuclear facilities in the decommissioning stage to reduce the cost of final disposal and volume of secondary waste.
Key words: decontamination, stainless steel, cerium, electrochemical, oxidization
INTRODUCTION
Decontamination of stainless steel contaminated with radionuclides is difficult because of its insolubility in general decontamination solutions. The dissolving rate of stainless steel in a nitric acid solution with cerium (4) will increase obviously, so that a thin layer of the surface of contaminated stainless steel will be removed rapidly within a few hours. The contaminants adhered strongly on the surface of the contaminated stainless steel will also be removed into the decontamination solution. Therefore, the contaminated stainless steel is decontaminated successfully.
The cerium(4) ion will be reduced into cerium(3) ion in the decontamination process by the reaction with the metal or metal oxides, and the cerium(3) ion can be re-oxidized into cerium(4) ion by the electrochemical method. Therefore, the decontamination solution can be used again and the secondary waste solution will be reduced. Some advantages of the electrochemical oxidization method are that no other materials are being brought into the decontamination solution, and the volume of secondary waste solution does not increase.
A variety of research work has been accomplished in America, Japan, France, and other countries, and the decontamination effect of this method has been widely confirmed. The purpose of our experiments is to select the proper ranges of operation parameters to achieve a suitable dissolving rate of stainless steel and regenerating rate of cerium(4) ion, so the decontamination process can be maintained and finished simply and successfully.
EXPERIMENTAL METHODS AND APPARATUS
Experimental Methods
The results of experiments for dissolving stainless steel was derived from measuring weight-loss and observing the status of the surface of the stainless-steel sample. Experimental conditions include the concentration of cerium(4) ion, concentration of HNO3 acid in the decontamination solution, and the temperature of the solution.
To obtain a suitable regeneration rate of cerium(4) ion, the issue was how to design a proper electrolytic cell so that the main product of the electrolytic process would be the cerium(4) ion. In our experiments, various types of electrolytic cells were designed and compared to each other to select the most suitable one for production and regeneration of cerium(4) ion.
Experimental Apparatus
The experiments for dissolving the stainless steel are performed in a 250 ml flask that is heated and agitated with an electromagnet agitator. The stainless-steel sample is 20x20x1 mm of 1Cr18Ni9Ti.
The cerium(4) ion is produced on the surface of an anode of an electrolytic cell in suitable operation conditions, but it can also be reduced into cerium(3) ion by the reducer in a Fe2+ ion solution or reduced on the surface of a cathode by obtaining an electron. Thus, the electrolytic cell must be designed to prevent the reducer from coming in contact with the oxidized cerium(4) ion, and prevent the cerium(4) ion from contacting with the surface of the cathode as much as possible.
The experiments for regeneration of cerium(4) ion are performed in a specially designed electrolytic cell, which apply the shielded cathode.
EXPERIMENTAL RESULTS AND DISCUSSIONS
Results of experiments include two parts: one is how to achieve the available dissolving rate of the stainless-steel sample, so that decontamination can be accomplished; and the second is how to achieve the acceptable regeneration rate of cerium(4) ion so that the effective decontamination process can be maintained.
The dissolving rate of stainless steel in nitric acid with the addition of the cerium(4) ion can be described as a function of concentration of Ce3+ ion, Ce4+ ion, HNO3; the temperature of the solution; and the flow status of the solution, as follows:
![]()
The parameter of flow status of the nitric acid solution cannot be described quantitatively in our experiments, so the function of Qs cannot be calculated with the parameter of flow status of the solution; however, for the dissolving rate, the flow status is an important factor. The results of our experiments are derived from a conservative condition of flow status.
The regenerating rate of Ce4+ ion is also the same situation and can be described as a function of concentration of Ce3+ ion and Ce4+ ion, the temperature of the solution, the flow status of the electrolyte in the cell, the status of electrodes, and the current density, as follows:
![]()
The flow status of the solution cannot be controlled as a quantitative parameter in our experimental conditions, but it is also an important factor to the regenerating rate, so the relation between function Qr and flow status Re cannot be described quantitatively in our lab experiments. We hope that the two functions can be figured out in the future when there is enough funding and time, so the operation parameters in the real decontamination process can be obtained from the two functions.
At present, the experiments involve measuring the dissolving rate at various concentrations of Ce3+ ion, Ce4+ ion, and HNO3; the temperature of the solution; the regeneration rate of Ce4+ ion at various concentrations of Ce3+ ion; and the temperature of the solution. In our experiments, the flow status of the solution was controlled at low-speed agitation, and not at strong convective status, thus the dissolving rate and regeneration rate at these conditions are lower than those at strong convective conditions. The experimental results of these phenomena are not included in this paper.
Results of Dissolving Experiments
Effect of Temperature
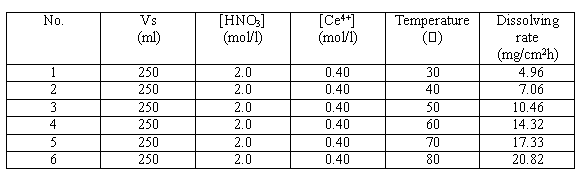
The temperature of decontamination solution ranges from 30· to 80· , and with the increase of temperature, the loss of weight from the sample increases obviously.
Table I. Effect of Temperature on Dissolving Rate of Stainless Steel
Effect of Concentration of Cerium(4) ion
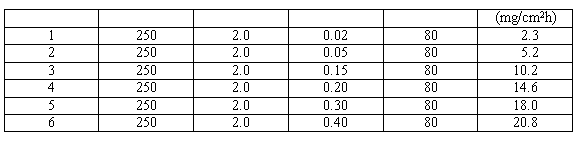
The concentration of cerium(4) ion is the main element to dissolve stainless steel. The concentration is in the range of 0.01 mol/l to 0.40 mol/l of Ce4+ ion. Weight loss of the sample increases with the increase of the concentration of Ce4+ ion at the same temperature of the solution.
Table II. Effect of Concentration of Ce4+ Ion on Dissolving Rate of Stainless Steel
Effect of Nitric Acid Solution on Dissolving Rate of Stainless Steel
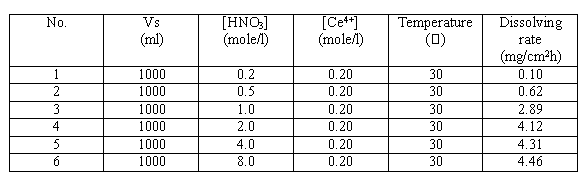
The contribution of nitric acid to the dissolving rate of stainless steel is small; this can be seen from various industrial data of erosion research. When at a certain concentration of Ce4+ ion, the change of concentration of nitric acid would affect the dissolving rate of stainless steel. The concentration of nitric acid of 2.0 mol/l is the recommended value.
Table III. Effect of Nitric Acid Solution on Dissolving Rate of Stainless Steel
Effects of other Factors
Factors that may affect the dissolving rate of stainless steel, in addition to those factors listed above, also include the condition of the surface of the sample. This is a complicated factor because there are a variety of contaminants adhering to the surface of stainless-steel equipment; their compositions and adhesion varies with the operating conditions commissioned in nuclear industry. In our laboratory, it is difficult to simulate all the conditions of contaminated stainless-steel, so the compositions of the decontamination solution should be adjusted slightly after the analysis of special compositions and contaminants on stainless-steel equipment to be decontaminated.
The condition of the stainless steel surface is smooth and even after dissolving in nitric acid solution with the Ce4+ ion.
Regeneration Rate of Cerium(4) Ion Oxidized by Electrochemical Method
The regenerating rate of cerium(4) ion is calculated by the following representation:
![]()
where:
[Ce4+]o is the concentration of Ce4+ ion in solution after electrochemical oxidization, (mol/l)
[Ce4+]i is the concentration of Ce4+ ion in solution before electrochemical oxidization, (mol/l)
Ve is the volume of solution, (l)
t is the time of electrochemical oxidization, (s)
Sa is the area of anode surface, (m2)
The anode was platinum plate or titanium net-plated with platinum and the cathode was platinum wire in our experiments [1].
The regeneration experiments were performed with two types of solutions of 2.0 mol/l HNO3 and 0.5 mol/l H2SO4. Both can be used as decontamination solutions, but the result of the dissolving rate in H2SO4 acid solution is not included in this paper.
Effect of Current Density
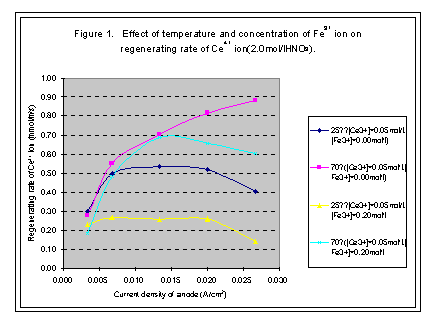
When concentration of Ce3+ ion is certain, the regeneration rate of Ce4+ ion increases with the current density of anode; however, it would not increase after arriving at a high point. The high point is named as limited current density and the regeneration rate at this point is called limited regeneration rate (see Figures 1, 2, 3, and 4).
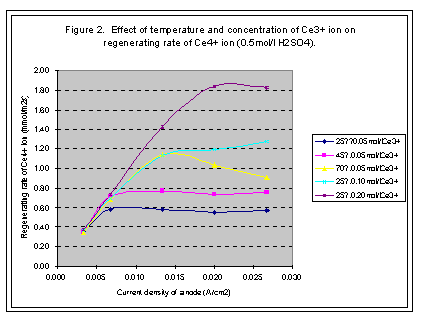
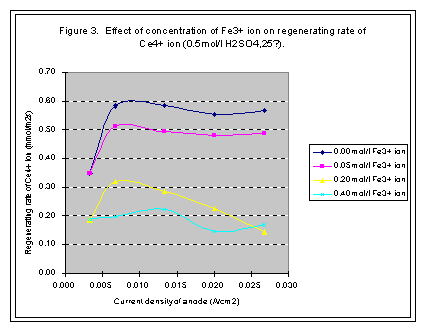
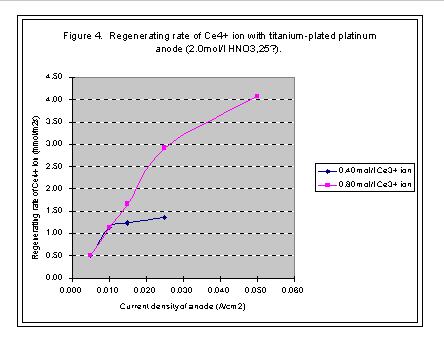
Effect of Temperature
In our experiments, the temperature of electrolyte varies from 25· to 70· . The limited regeneration rate of Ce4+ ion produced in the cell increases with the increase of temperature of the solution (see Figure 1).
Effects of Concentration of Cerium(3) Ion
The concentration of Ce3+ ion is from 0.05 mol/l to 0.8 mol/l in our experiments. The limited regeneration rate of Ce4+ ion increases with the increase of the concentration of Ce3+ ion in solution. When concentration of Ce3+ ion is lower than the 0.2 mol/l, the limited regenerating rate of Ce4+ ion is very small with titanium net-plated with platinum anode, but with platinum plate anode, the regenerating rate and current efficiency is acceptable when the current density is small enough. If the concentration of Ce3+ ion is higher than 0.2 mol/l, then the titanium net-plated with platinum anode can satisfy the requirements of regenerating Ce4+ ion. The current density can be as high as 0.025A/cm2 and the current efficiency can be higher than 90% (see Figures 2 and 4).
Effect of the Concentration of Fe3+ Ion
The concentration of Fe3+ ion will increase in the decontamination process, and can be reduced on the surface of cathode of the electrolytic cell. The reducer of Fe2+ ion will react with Ce4+ ion, so with the increase of Fe3+ ion, the consuming rate of Ce4+ ion will increase (see Figures 1 and 3).
Effects of Other Factors
Elevated temperatures will increase the current conductivity of the solution, so the voltage will be smaller than that at the lower temperature when experiments are at the same current density.
At high temperature, the capability of erosion and oxidization of the decontamination solution to container materials is strong, thus the amount of reduction of cerium(4) by contacting with the container material increase. Therefore, if the common materials are used as container material (such as polyvinyl chloride and polyethylene), the solution should be at lower temperatures.
Recommended Parameters for Real Decontamination Process
The dissolving rate of stainless steel at the conditions of 0.01 mol/l and 30· is the lowest in our experiments, about 0.36 mg/cm2h. The highest is about 17.33 mg/cm2h at conditions of 0.4 mol/l and 70v. Both experiments were conducted under general electromagnet agitation conditions. When at 0.13 mol/l and 30· , the dissolving rate is about 0.96 mg/cm2h.
When the temperature of the solution is 30· and the concentration of Ce3+ ion in electrolyte is 0.4 mol/l, the generating rate of Ce4+ ion can be as high as 4.26 mmol/m2s. If the total area of anode of an electrolytic cell is 1 m2, the electrolytic cell can produce 15.34 mol Ce4+ ion per hour. When the dissolving rate of stainless steel is maintained at 0.96 mg/cm2h (about 1.2· m/h), the consuming rate of Ce4+ ion for an area of 1 m2 contaminated stainless-steel is about 0.51 mol/h, an electrolytic cell of 1 m2 anode area can maintain 29.8 m2 of stainless steel dissolved at the rate of 0.96 mg/cm2h. With the increase of Fe3+ ion, the regenerating rate of Ce4+ ion will decrease, thereby producing the dissolving rate of stainless steel.
The above calculation is our basic recommendation. In a practical application of the decontamination process, if a high dissolving rate is needed, the temperature of the solution can be elevated, the concentration of Ce4+ ion can be increased, and the strength of agitation can be enhanced. If a high regenerating rate of Ce4+ ion is needed, the temperature of the solution can be elevated, the concentration of Ce3+ ion can be increased, and the strength of agitation can be enhanced.
CONCLUSIONS
Temperature of the decontamination solution and the concentration of Ce4+ ion are the prime factors that affect the dissolving rate of stainless steel in nitric acid solution containing Ce4+ ion. The contaminated stainless steel can be dissolved at a certain rate by controlling these two factors.
The suitable regenerating rate of Ce4+ ion can be achieved by the anode of titanium plated with platinum at a specific concentration of Ce3+ ion, temperature and specific current density in a specially designed cell, so the decontamination process can be maintained.
REFERENCES