PLASMA PROCESS FOR SYNTHESIS OF DISPERSED
URANIUM OXIDE FROM NITRIC ACID SOLUTION
Alexander V. WANOV, Evgeny A. FILIPPOV, Vyacheslav M. ABASHKIN
Russian Research Institute of Chemical Technology
33 Kashirskoe Ave. Moscow 115230
Tel: (095) 324-5461; FAX: (095) 324-8869; E-mail: vs@khimcon.msk.ru
Yury N. TUMANOV
Russian Scientific Center "Kurchatov Institute"
Moscow
ABSTRACT
Oxide materials find increasing applications in various fields of modem technique. It is well known a role of uranium in nuclear energetics. We suggest practically universal method for synthesis of numerated oxides (uranium oxide in the first place) and oxide compositions based on plasma decomposition of disintegrated solutions of metals.
The nitrate solution of the metal (or metals) is dispersed by a sprayer into the air plasma flow. The solution drops mix with plasma and convert to oxides of dissolved metals, water vapor, nitrogen oxides, nitrogen, oxygen.
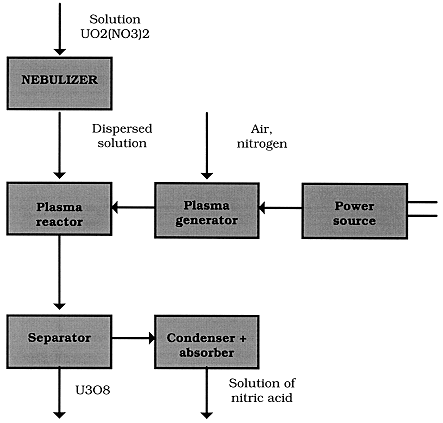
The specific elements of the flow sheet are the plasma generator (a plasma torch and a power source), plasma reactor, separator of powder and gas.
The main part of the plasma apparatus for manufacturing disperse oxide of uranium from nitrate solution is a plasma reactor. Mixing the solution with plasma is achieved due to the formation of a cone of the disintegrated solution descending on the plasma flow and penetrating it; the solution drops entering the plasma flow are decelerated or accelerated.
Disperse and gaseous phases are separated with using filters. Disperse materials must be given off from the flow under conditions when all the recombination processes are thermodynamically forbidden or kinetically slowed down.
After separator gas flow is directed into a condenser, where water vapor is condensed and nitrogen oxides are absorbed partly. The condensate obtained is used for total absorbing nitrogen oxides from the gas flow. The offered method allows to obtain practically all metal oxides from their nitrate solutions with unique physical and chemical properties.
INTRODUCTION
The nitrate solutions and melts are basic products of the hydrometallurgical processing in nuclear industry. Principal scheme of industrial production of metal oxides includes the following stages: precipitation of metal salt, filtration, drying, calcination of the salt, reprocessing (or dumping) of the raffinate, sorption of the off-gas (nitrogen oxides). The plasmachemical approach to the production of metal oxide is based upon the disintegration of initial metal solution and its injection to the plasma reactor, where the dispersed solution sharply boils up to dryness followed by instant high temperature heating and production of the metal oxides and off-gases (water vapor, nitrogen oxides and oxygen).
A plasmachemical decomposition of the UO2 (NO)3 - solution is generally described by overall reaction:
| UO2 (NO3)2*6 H20® U3O8 + 2 NO2 + 4 NO + 4 02 + 6 H20 | (1) |
The principal scheme of the process is shown in Fig. 1.

Figure 1. Schematic Diagram of Obtaining Oxide Materials by Plasma Decomposition of Nitric Solutions of Metals
The complex apparatus scheme compiles the following units:
Decomposition of the drops inside the reactor is shown in Fig.2. At first the drops are heated up to the boiling point and partially dried; the second stage is the complete water evaporation; and finally - overheating and decomposition of the salt residual.
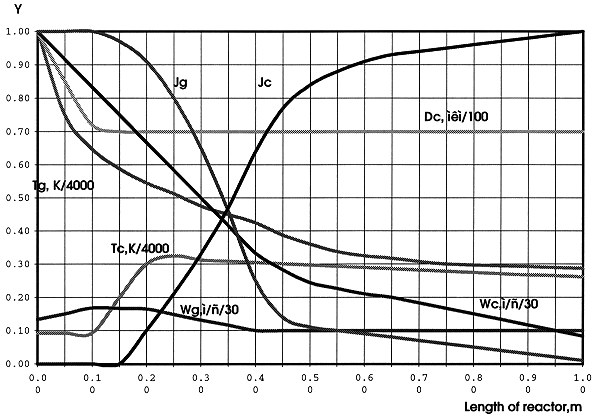
Figure 2. Change of Process Parameters at the Decomposition of Uranium Nitrate Solution in Air Plasma Heat Carrier
The development of the optimum regime for plasma reprocessing is based upon the modeling of plasma reactor, also as on calculation and designing of its geometry -primarily the ratio of its length, L, and cross diameter, D - to achieve the determined direction and extent of decomposition of the source solution. Another principal requirements, as usually, are: low energy consumption and high coefficient of efficiency.
RESULTS AND DISCUSSION
The following parameters were chosen to model the plasma converter:
| electric power of the plasma torch (PT), kW | 100-4000 |
| start PT temperature, K | 4000-6000 |
| start drop diameter, m | 20-200 |
| start speed of the drops coming to reactor chamber (Wk), m/s | 30-300 |
| consumption of source solution, kg/s | 0,015-0,23 |
| uranium concentration in feed solution, g/1 | 50-500 |
Main results of the modeling are shown in Fig.3. The Figure shows the changing parameters of plasma heat-carrier and distributed in it disperse phase while driving of a two-phase stream along the reactor, for uniform dispersion of the feed solution into air/plasma heat-carrier. These dependencies allow to define the length of the reactor providing the desirable chemical composition, final temperature of heat-carrier, speed of gaseous and condensed stream, diameter of a final particle, supposing that secondary and consequent subdivision does not happen. Overall time of complete conversion may be estimated too.
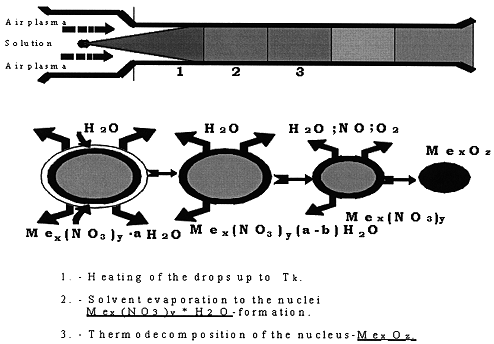
Figure 3. Thermodecomposition of the Solution Drops
The zone of complete decomposition of uranyl nitrate approximately to 99% has a length of 0.95 m. A temperature of heat-carrier inside the plasma torch dramatically reduces while water evaporation, and increases a little with the torch diameter. The calculation has shown, that a length of evaporation zone rises with growth of an initial velocity of the drops, Wk, and their size, Dk.
We used the results of simulated modeling based on the above-stated relations to design the plasma reactor for production of powdery oxide materials. An adequacy of the determined model was checked up experimentally. The test was carried out using an integrated technical parameters of the reactor and plasma process, indicated in Table I.
Table I. Comparison of Model Calculations with Experimental Results for
Decomposition of Uranyl Nitrate Solution
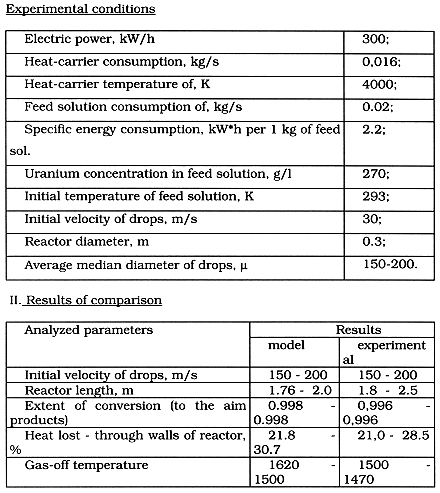
The data show that the error in determination the integrated parameters of plasma conversion process is less than 20%, approving an adequacy of the developed mathematical model.
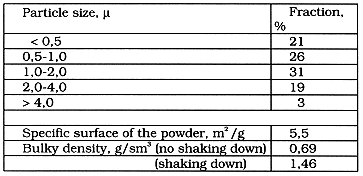
The experimentally produced batches of powdery uranium oxide have the following size distribution (see Table II).
Table II. Distribution of UOx Particles in Size
CONCLUSIONS