TREATMENT OF EBR-I NaK MIXED WASTE AT ARGONNE NATIONAL LABORATORY AND SUBSEQUENT LAND DISPOSAL AT THE IDAHO NATIONAL ENGINEERING AND ENVIRONMENTAL LABORATORY
S. D. Herrmann, J. A. Buzzell, M. J. Holzemer
Argonne National Laboratory-West
P.O. Box 2528
Idaho Falls, ID 83403-2528
ABSTRACT
Sodium/potassium (NaK) liquid metal coolant, contaminated with fission products from the core meltdown of Experimental Breeder Reactor I (EBR-I) and classified as a mixed waste, has been deactivated and converted to a contact-handled, low-level waste at Argonne's Sodium Component Maintenance Shop and land disposed at the Radioactive Waste Management Complex.
Treatment of the EBR-I NaK involved converting the sodium and potassium to its respective hydroxide via reaction with air and water, followed by conversion to its respective carbonate via reaction with carbon dioxide. The resultant aqueous carbonate solution was solidified in 55-gallon drums.
Challenges in the NaK treatment involved processing a mixed waste which was incompletely characterized and difficult to handle. The NaK was highly radioactive, i.e. up to 4.5 R/hr on contact with the mixed waste drums. In addition, the potential existed for plutonium and toxic characteristic metals to be present in the NaK, resultant from the location of the partial core meltdown of EBR-I in 1955. Moreover, the NaK was susceptible to degradation after more than 40 years of storage in unmonitored conditions. Such degradation raised the possibility of energetic exothermic reactions between the liquid NaK and its crust, which could have consisted of potassium superoxide as well as hydrated sodium/potassium hydroxides.
Treatment of the EBR-I NaK was a concerted effort between the Department of Energy C Idaho Operations and Chicago/Argonne Group West offices, Lockheed Martin Idaho Technologies Co., Argonne National Laboratory, and the State of Idaho Department of Environmental Quality. The mixed waste treatment was performed under a Consent Order between the Department of Energy and the State of Idaho and under a RCRA Part B Interim Status Treatment Permit for the modified Water Wash System in the Sodium Component Maintenance Shop.
Treatment of the EBR-I NaK was successful, yet it involved some unanticipated events and subsequent technical and administrative mid-course corrections. This paper documents these operational experiences to serve as lessons learned for potential future applications. The objectives of the NaK treatment project were accomplished by converting the mixed waste to a nonhazardous form and land disposing the treated waste.
BACKGROUND
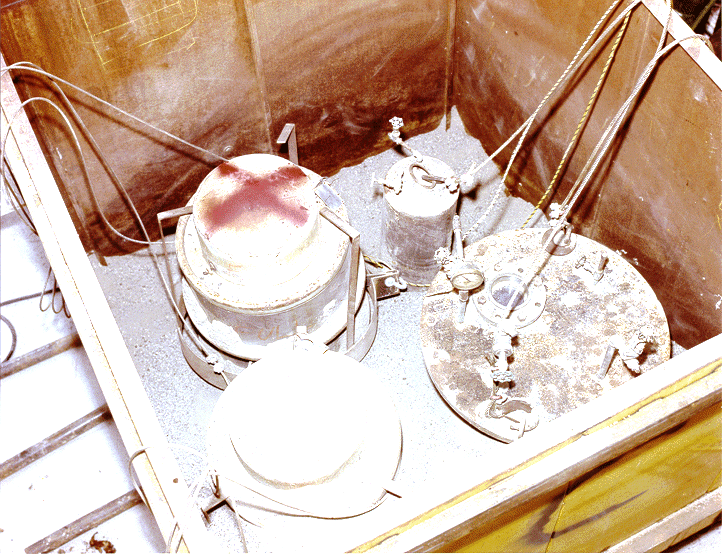
Physics testing was performed on the EBR-I Mark-II core in November of 1955. For these tests it was necessary to shut off the flow of the liquid metal NaK coolant to the reactor tank. The core was subjected to a power excursion, which resulted in the meltdown of approximately one half of the core's fueled region, consequently releasing fission products and fuel to the reactor tank. Additional details of the EBR-I experiment and analysis of the removed core have been documented.1 The core contained highly enriched uranium-zirconium alloy fuel rods clad with stainless steel. In addition, the core was loaded with a 10.5 gram experimental specimen of plutonium. In order to recover the core for analysis, it was first necessary to remove the NaK from the reactor tank. This process resulted in the containerizing of material in two drums and two closed pipe sections, which are shown in Figure 1. Due to insufficient analyses and material balances, complete recovery of the 10.5 gram plutonium specimen was not verified. Consequently, there existed a possibility that the fission product laden NaK contained a portion of the experimental plutonium.
The NaK containers, consisting of two 55-gallon Mine Safety Appliance (MSA) drums, one 60-gallon and another 10-gallon closed pipe section, contained a maximum potential material inventory of 180 gallons. These NaK containers were stored in an underground pit at the EBR-I complex from 1956 to 1973. Decommissioning of EBR-I resulted in the treatment of the remaining NaK primary coolant (approximately 5500 gallons) in 1972 at the Idaho National Engineering and Environmental Laboratory (INEEL).2 Although the contents of the four NaK containers could have likewise been processed, the option was not pursued based on implications regarding its potential plutonium content. Consequently, the NaK containers were transferred to another storage location on the INEEL, the Army Reentry Vehicle Facility Site (ARVFS) bunker. The containers were reexamined in 1979, after which they remained in the ARVFS bunker until retrieval for treatment in 1995.
Radiation surveys in 1973 revealed radiation readings on contact with the NaK containers of up to 7 R/hr. A gamma scan performed during the 1979 inspection revealed the majority of the gamma activity emitted from the containers to be cesium-137. Based on the 1973 and 1979 surveys and estimations from core fission product yields, 20 curies of radioactivity were estimated to be contained in the potential 180 gallons of NaK, consisting predominantly of cesium-137/barium-137m and strontium-90/yttrium-90.3 No sampling of the material in any of the four NaK containers had been performed since its inception and nearly 40 years of storage, nor was it sampled directly prior to the subject treatment.

Fig. 1. EBR-I NaK Containers at ARVFS Bunder (1979)
TREATMENT PROCESS DESCRIPTION
Collaboration toward the removal and treatment of the four NaK container contents began in earnest in 1995 between Lockheed Martin Idaho Technologies Company (LMITCO), Department of Energy (DOE) C Idaho Operations and Chicago/Argonne Group West offices, and Argonne National Laboratory-West (ANL-W). ANL-W had experience and facilities to safely handle alkali metals, largely as part of its operation and maintenance of the sodium cooled EBR-II. The treatment of the EBR-I NaK was accommodated with minor modifications to the Water Wash System within the Sodium Component Maintenance Shop (SCMS). The objective in treating the EBR-I NaK was conversion of this mixed waste to a nonhazardous form and subsequent land disposal at the INEEL Radioactive Waste Management Complex (RWMC). This mixed waste treatment was subject to State and Federal Environmental Protection Agency requirements. The planned treatment process involved 3 parts: 1) Deactivation of the NaK; 2) Carbonation of the resultant aqueous hydroxide solution; and 3) Immobilization of the resultant aqueous carbonate solution. This process also included treatment of the residual contents within each container.
NaK Deactivation
NaK deactivation involved transferring the mixed waste from its storage containers and reacting the alkali metal with air and water in the Water Wash System as illustrated in Figure 2. The following describes the planned sequence of events for treatment of a typical NaK container. A NaK container was placed in a shielded overpack, covered with a glovebox containment and the atmosphere inerted with argon. Connections were made to a NaK container's existing fittings and the internal atmosphere of the vessel was purged with argon. After a dip tube was inserted concentric to the container's existing drain pipe, a vacuum was placed on a pressure- and vacuum-rated feed tank and, along with a slight over pressure (0-2 psig) on the NaK container, the NaK was vacuum transferred into the feed tank. With the contents removed, the empty NaK container was disconnected from the feed tank. The vacuum on the feed tank was replaced with a positive argon pressure, up to 25 psig, to drive the NaK into a burn pan within the water wash vessel. At a rate of up to 50 lbs/hr, the NaK was reacted with air and water, forming sodium and potassium oxide and hydroxide, respectively, by the following reactions.
|
|
(1) |
|
|
(2) |
The sodium and potassium hydroxide solution drained to the scrubber water tank. Sodium and potassium oxide vapors were forced through a wet scrubber via a 3000 cfm air flow, in which the oxides reacted with water to form a sodium and potassium hydroxide solution by the following reactions.
|
|
(3) |
|
|
(4) |
By this process a typical NaK container produced approximately 200 gallons of 25 wt% sodium and potassium hydroxide solution.
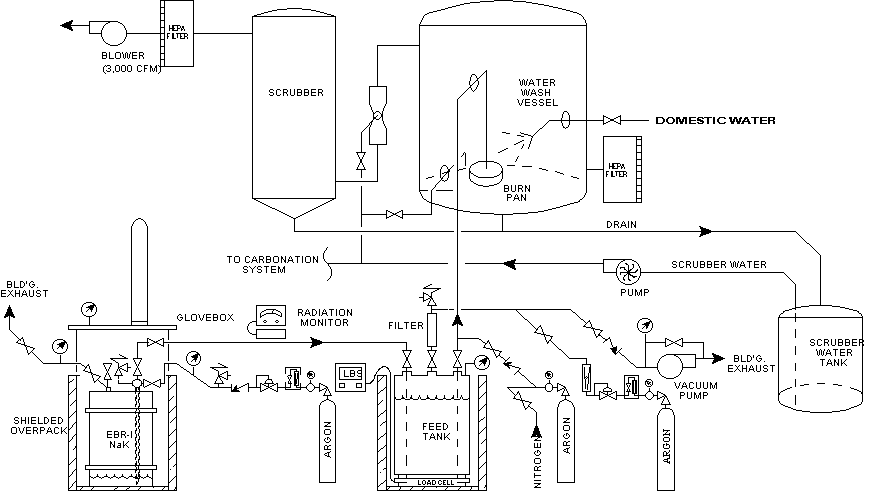
Fig. 2. Feed and Transfer System for EBR-I NaK Deactivation
Carbonation
The carbonation step of the treatment process involved converting the sodium and potassium hydroxide to a sodium and potassium carbonate solution via reaction with carbon dioxide. The configuration of equipment to perform the carbonation step is illustrated in Figure 3. Sodium and potassium hydroxide solution in the scrubber water tank was recirculated up through the bottom of a carbonation vessel at a rate of approximately 1 gpm and contacted with finely divided gaseous carbon dioxide, introduced through a sintered metal sparge element of 10 mm porosity. The carbon dioxide reacted with the sodium and potassium hydroxide solution via the following overall reactions.
|
|
(5) |
The recirculated solution gravity drained from the carbonation vessel back to the scrubber water tank. A combination of the mass of carbon dioxide delivered and the carbonation vessel pressure and temperature provided an indication of hydroxide to carbonate conversion completion. Sampling and analysis from the scrubber water tank confirmed the conversion of hydroxide solution to a carbonate solution of pH less than 12.5. Analysis also provided determination of plutonium and toxic metal concentrations.
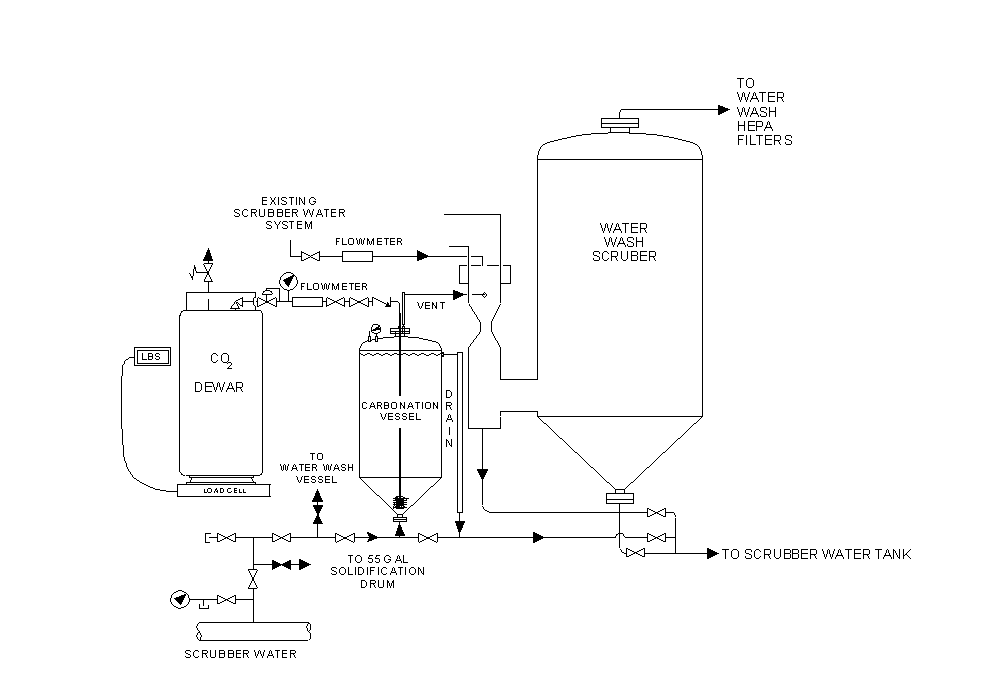
Fig. 3. Carbonation System for EBR-I NaK Treatment Process
Immobilization
Immobilization of carbonate solution involved solidification with Aquaset II-H (a product of Fluid Tech, Inc.) within 55-gallon drums. A solidification system, as illustrated in Figure 4, was configured inside a contamination controlled work tent. A polymer sleeve connected a glovebox containment to a drum, which was located within a shielded overpack. Thirty-two to 37 gallons of sodium and potassium carbonate solution were pumped from the scrubber water tank to a drum. Up to 220 lbs of Aquaset II-H was slowly added to the carbonate solution as it was being stirred. Once the predetermined amount of Aquaset II-H was added and a proper consistency was obtained, the mixer was stopped and samples were taken as necessary. The mixer impeller was driven to the bottom of the drum and decoupled from the mixer. The sleeving between the drum and glovebox was heat-sealed and cut, decoupling the containment from the drum. The drum was fitted with a lid, removed from the overpack, and placed in shielded storage. The solidified carbonate solution was vented through HEPA filtration as it cured for a minimum of seven days before the drums were sealed. Likewise, samples taken of the treated material were allowed to cure prior to isotopic analysis and metal concentration determination.
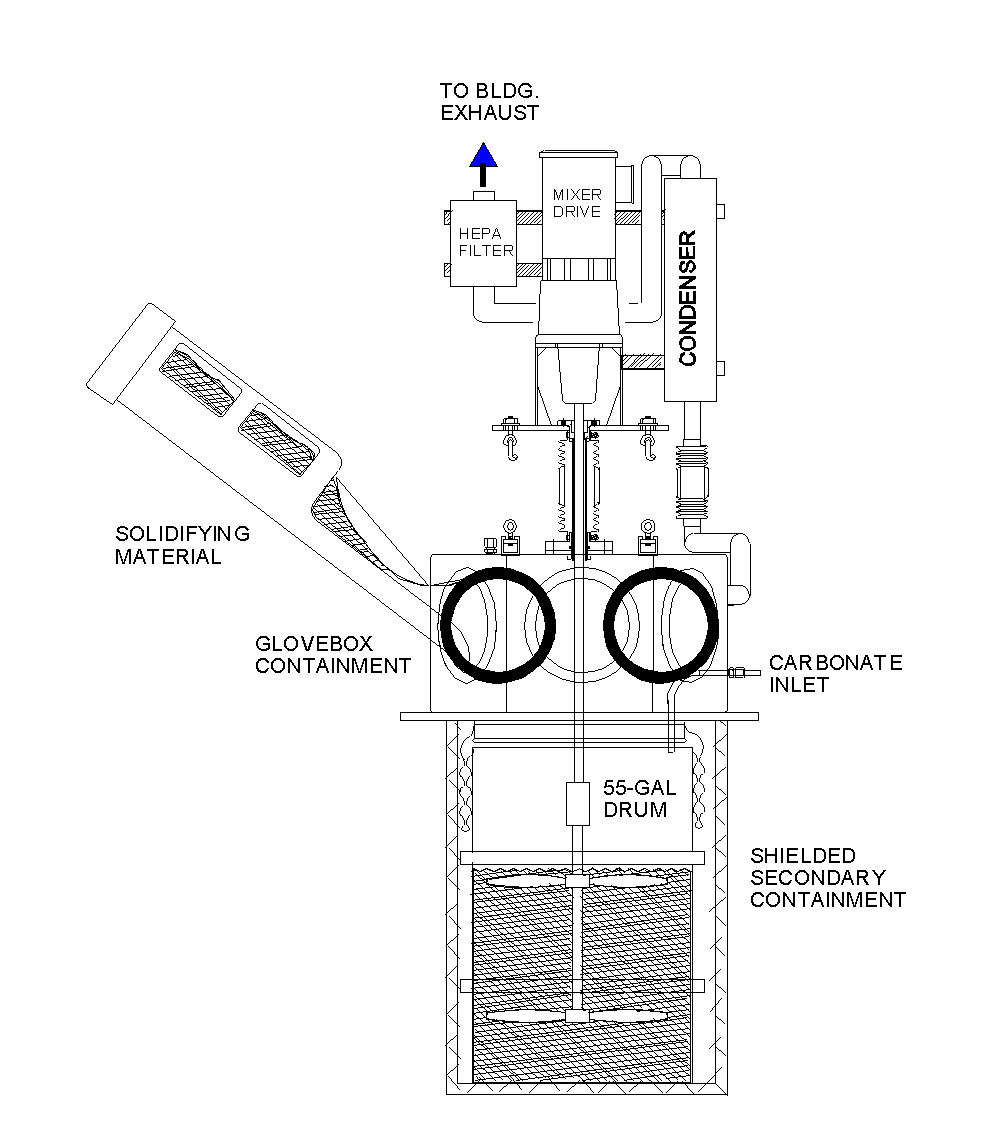
Fig. 4. Solidification System for EBR-I NaK Treatment Process
Treatment of Residuals
The steps for NaK deactivation, carbonation, and immobilization were performed in succession for the NaK containers, each producing six to seven 55-gallon drums of solidified waste. The treatment process was applied to the MSA containers first, followed by the 60-gallon vessel and lastly the 10-gallon container. Following the last deactivation of bulk NaK and subsequent carbonation, and prior to the final batch of waste immobilization, the residual contents of each container were treated. Less than one inch of NaK remained in each container after it was emptied. To treat this residual each container was placed inside the water wash vessel, inerted with nitrogen, steamed, and then rinsed with carbonate solution from the scrubber tank. Finally, the container was opened and visually inspected for absence of material and radioactive swipe surveys were taken.
RECOGNIZED CHALLENGES
In addition to management of the known reactive nature of NaK and radiation levels, many of the challenges posed by the NaK treatment revolved around the uncharacterized nature of the material. Specifically, the degree of NaK degradation, the plutonium content, and the toxic metal concentrations were unknown. The decision was made to proceed with the NaK treatment in spite of the material's incomplete characterization, largely due to the risks involved in attempting to obtain a representative sample. It was also understood that sampling the NaK would be nearly identical to the steps required for its treatment. Consequently, the treatment process needed to take precautions against and provide contingencies for the incomplete waste characterization.
NaK Degradation
After 40 years of storage in unmonitored conditions, it was perceived as probable that an inert cover gas no longer existed within the NaK containers and that thermal cycling of the containers induced atmospheric exchange. The potential introduction of air into the containers raised the possibility of potassium super oxide formation and subsequent reaction with the NaK. Previous investigations suggested that agitation of a NaK container with a potassium superoxide surface crust or penetration of a superoxide crust, causing contact between the NaK and the superoxide, could induce a condensed phase exothermic reaction.4 It was proposed that within a closed container, such a reaction could lead to a breach of containment and/or NaK ejecta. Furthermore, the possibility existed that a hydrogen explosion upon agitation of the NaK containers could occur, based on reported spontaneous combustion of stored alkali metal containers. If the exchange of atmosphere in the NaK containers had progressed such that moisture laden air was introduced, the moisture would have reacted with the NaK to form hydroxides and hydrated hydroxides. Disturbances of a surface of hydroxides and hydrated hydroxides and subsequent reaction with the NaK could have liberated hydrogen gas with energy sufficient to ignite the hydrogen within an enclosed air atmosphere. The resolution for these perceived risks was intentional remote agitation of the NaK containers prior to treatment, encapsulation of the NaK container within an inert atmosphere, initial purging of the container atmosphere with argon, and remote insertion of the dip tubes into the NaK containers.
Plutonium
Although the 10.5 grams of plutonium contained in the melted portion of the EBR-I core were unaccounted for and were, therefore, possibly present in the NaK, it was recognized that plutonium has little solubility in alkali metal.5 Therefore, if present, the plutonium would most likely have been in precipitant form in the NaK and would have remained so in hydroxide and carbonate solutions. Part of the solution for managing the risk of plutonium was to assay each container externally prior to treatment. Furthermore, if plutonium were detected in the water wash vessel scrubber water tank, equipment and techniques were prepared to mechanically filter and capture the plutonium.
Toxic/Hazardous Metals
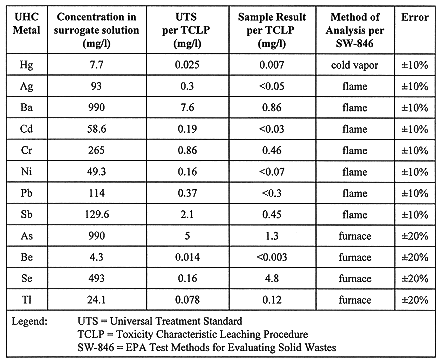
Without prior characterization of the NaK and per Resource Conservation and Recovery Act (RCRA) management of the NaK treatment, the position was taken to sample and analyze the solidified treated waste for 12 Underlying Hazardous Constituent (UHC) metals. In order to verify the performance of metal retention by Aquaset II-H a surrogate sample of a UTS metal laden carbonate solution was prepared, solidified, allowed to cure for 7 days, and analyzed per SW-846 techniques. The results are illustrated in Table I. With this information, if metals appeared in the scrubber water solutions, their concentrations could be compared against the performance criteria and a determination made on how to proceed with waste solidification.
Table I. Performance of Underlying Hazardous Constituents (UHC) Metal Retention by Aquaset II-H in Surrogate Carbonate Solution from EBR-I NaK Treatment
OPERATIONAL EXPERIENCE
There were two major activities involved in handling the EBR-I NaK mixed waste treatment: 1) Retrieving the NaK containers from the ARVFS bunker and transporting them to ANL-W; and 2) Treating the NaK in the Water Wash System and disposing of the treated waste at the RWMC. The following summarizes the operation and highlights some of the significant events.
NaK Transportation
In September of 1995 LMITCO personnel removed the EBR-I NaK containers from the ARVFS bunker. The NaK containers within the dumpster were remotely agitated and no hydrogen or other indications, which would suggest an exothermic reaction, were detected. Radiological surveys of the containers revealed on-contact radiation levels of up to 4.5 R/hr. Plutonium assays of each container were performed, which technique examined prompt neutrons from the spontaneous fission of Pu-240, and revealed less than detectable levels, i.e. less than 1 gram of plutonium per container.6 The containers were packaged within a shielded cask and an unprecedented closure of a 12-mile portion of public highway allowed transportation of the mixed waste from the Central INEEL facilities to ANL-W.
NaK Treatment
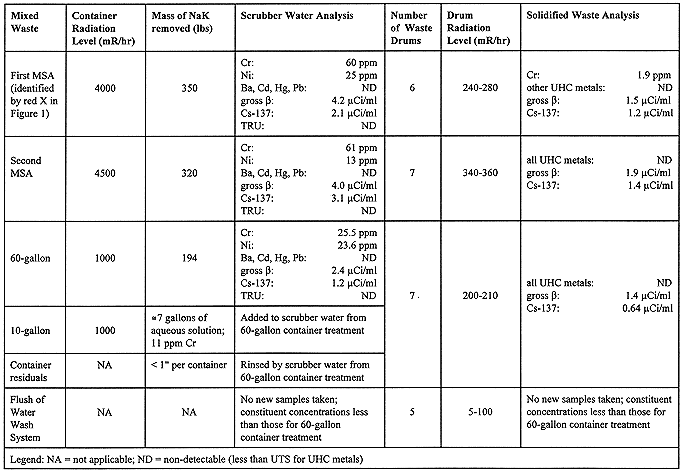
Treatment of the NaK in the SCMS commenced as originally planned. Summary results are presented in Table II. The following describes some of the challenges and unanticipated events encountered during the NaK treatment.
Little degradation of the NaK was identified. There was only one occurrence of NaK solids buildup on the first MSA container vent pipe. Inerting the container, via this vent pipe, proceeded after an argon fill tube was bored through the solid material. No NaK degradation products were encountered upon insertion of dip tubes into the NaK. In fact, shiny metallic NaK was visually observed on the surface of each of the three large containers, as the dip tubes were inserted.
Following treatment of the first MSA container contents, chromium and nickel were detected at 60 and 25 ppm, respectively, in the scrubber water solution. This chromium to nickel ratio was consistent with the 18:8 chromium to nickel ratio of common stainless steels. Since the chromium and nickel concentrations were well within the performance capability of Aquaset II-H, as identified in Table I, solidification of the treated waste continued. TCLP of the solidified material, randomly sampled from one of the six waste drums, revealed a chromium concentration of 1.9 ppm. According to the Aquaset II-H manufacturer, such an appearance of chromium was indicative of its hexavalent form, which was capable of being reduced by Fe(II). As chromium appeared in subsequent samples, ferrous sulphate heptahydrate (FeSO4.7H2O) was added to the carbonate solution at a 60:1 iron to chromium ratio as part of the immobilization process. Subsequent analysis of solidified samples resulted in non-detectable levels of chromium, as well as the other prescribed metals.
Prior to treatment of the 10-gallon container contents, the vessel was radiographed to identify its internal piping configuration, which revealed an existing dip tube within the container. This radiography also revealed that the container was approximately two-thirds full. Due to this small volume, the NaK from the 60-gallon container was retained in the feed tank so that the expected NaK in the 10-gallon container could be added to it. In contrast to the three large NaK containers, transfer of the 10-gallon container contents utilized the container's existing dip tube and, therefore, did not require insertion of a separate tube. Upon starting the transfer of the 10-gallon container contents to the feed tank, operators recognized that a reaction was occurring in the transfer line between the two vessels and immediately stopped the transfer. Sampling and analysis of the liquid within the 10-gallon container determined that it was water with a pH greater than 13 and contained 11 ppm of chromium. It exhibited no ignitable characteristics, nor did it contain organic constituents. The liquid from the 10-gallon container was subsequently admitted into the scrubber water tank and combined with the carbonate solution resultant from the 60-gallon container NaK treatment.
Following the treatment of the bulk NaK, the residual NaK within the containers, and subsequent solidification of the scrubber water, the water wash system was rinsed and flushed. The water accumulated from this rinse and flush was likewise solidified and added to the total treated waste volume. Following waste characterization and shipment preparations, the twenty-five 55-gallon drums of treated waste were transported and land disposed at the RWMC in September 1996.
Table II. Summary Results of EBR-I NaK Treatment
WASTE DISPOSAL AND RCRA MANAGEMENT
In accordance with RCRA requirements for the EBR-I NaK treatment, ANL-W developed interim status tank and container treatment documentation. This documentation identified that the treatment standards would be the Universal Treatment Standards (UTS) for UHC. Land Disposal Restriction (LDR) treatment requirements for a reactive waste (D003) with the possible existence of toxic characteristic (TC) metals, prior to LDR Phase III final rule, was deactivation and treatment standards associated with the TC metal waste codes (D004 - D011). Treatment of UHC to UTS was not a current requirement. ANL-W's decision to treat the NaK waste to the conservative UTS for UHC was based on: 1) the incomplete characterization of the waste; 2) the inconsistency in treatment standards for characteristic wastes; and 3) ANL-W's commitment to minimizing effects on the environment.
The UHC listed in 40 CFR 268.42 were evaluated for reasonably expected hazardous constituents. The probability that UTS listed organic compounds would exist in NaK exposed to operating reactor environments was extremely small. This statement was based upon the fact that the primary role of alkali metal in alkali-metal-hydrocarbon reactions was that of a strong reducing agent. Furthermore, this assumption was supported by ANL-W chemical analyses which showed the carbon content in sodium metal to be very low. Therefore, inorganics were the only constituents considered. Of the 19 inorganic constituents identified in 40 CFR 268.48 UTS, two were not underlying hazardous constituents in characteristic waste (zinc and vanadium), two others did not have finalized treatment standards in nonwaste water form (fluoride and sulfide), one was not applicable to the waste (mercury retort residues), and two were unable to exist in the systems where the waste was generated (cyanide, total and amenable). Therefore, the reasonably expected hazardous constituents having a potential to be in the waste were the remaining 12 inorganic constituents C mercury, silver, barium, cadmium, chromium, nickel, lead, antimony, arsenic, beryllium, selenium, and thallium.
Other than developing interim status documentation and determining which treatment standards would be implemented for the NaK waste, the Water Wash System within the SCMS needed only a minor modification to comply with RCRA requirements for tanks. This modification was to provide an impermeable surface for secondary containment under the water wash vessel and the scrubber water tank.
A primary concern was the disposal of the waste after deactivation. Finding an appropriate disposal facility and ensuring the facility's disposal criteria could be met were two major activities which had to be completed before treating the EBR-I NaK waste. The INEEL RWMC was identified as an appropriate disposal facility. The packaging of the waste for disposal was determined to be independent of the analytical results obtained after treatment. Solidification would be performed, regardless of the presence or absence of heavy metal contamination, due to criteria requirements at the RWMC. Disposal criteria at the RWMC did not allow liquids to be land disposed.
Since deactivation of the EBR-I NaK revealed that the first six drums sampled and analyzed failed to meet the UTS for UHC for chromium, an evaluation for additional treatment of these six drums to meet UTS was conducted. The concern at this point in the treatment process was exposing operators to additional radiation. It was decided that since the six drums did meet currently specified LDR treatment standards, unnecessarily exposing personnel to additional radiation to meet the conservative treatment standard was not warranted.
LESSONS LEARNED AND DISCUSSION
Lessons learned from treatment of the EBR-I NaK stemmed from the incomplete characterization of the mixed waste, in spite of precautions taken for potential hazards. Specifically, these lessons included the minimal degradation of the NaK, the negligible presence of plutonium, the recourse for chromium containing waste, and the heightened sensitivity towards waste characterization prior to treatment as a result of the water content in the 10-gallon container.
Degradation of the NaK after 40 years of storage was not nearly as severe as anticipated. Buildup of solid NaK products in the first MSA vent pipe was the only perturbation to the process as a result of material degradation. Such buildup, however, was identified as consistent with the nature of NaK to encapsulate itself by sealing off possible container leak sites.
Although the possibility of plutonium contamination in the NaK existed, the probability was viewed as low, due to the lack of plutonium solubility in the alkali metal. The external plutonium assays of the NaK containers, although limited in sensitivity, provided confidence to proceed with the NaK treatment. Subsequent analyses during the treatment process confirmed the absence of any significant quantities of plutonium and no additional controls for plutonium containment were implemented.
The differentiation of behavior between Cr(III) and Cr(VI) in the solidification process was not examined in the pretreatment surrogate testing, nor would the presence of Cr(VI) have been expected in the strongly reducing environment of NaK. Nevertheless, the ferrous sulfate heptahydrate in sufficient quantity did prove effective in allowing the Aquaset II-H to immobilize chromium.
The inadvertent reaction of NaK and water was potentially the most significant incident in the treatment process. NaK-water reaction products in the flexible metal transfer line cut off continued flow of water and subsequent reaction without any serious consequence. There were no indications of NaK-water reaction within the feed tank. Although the function of the 10-gallon container in the original NaK removal from the EBR-I reactor tank was questioned C thereby prompting the radiography of the container in order to assess its internals C whether it contained something other than the historically documented NaK was not considered. Therefore, the obvious lesson learned from this incident was to sufficiently characterize the waste prior to treatment. Of the four mixed waste containers, the 10-gallon container was the only container in which visual observation of the NaK prior to removal was unobtainable, since transfer of the 10-gallon container contents did not involve insertion of a separate dip tube.
CONCLUSION
Treatment of the EBR-I NaK concluded the 40-year storage of a legacy mixed waste at the INEEL. The objectives of the treatment project were accomplished by converting the mixed waste to a nonhazardous form and land disposing the treated waste at the RWMC. Technical expertise, effective management, and commitment by many individuals contributed to the successful treatment of the EBR-I NaK.
ACKNOWLEDGMENTS
The authors wish to acknowledge the following individuals for their significant contributions in the EBR-I NaK Project: Charlie Dietz of LMITCO for his lead role in managing the project and transportation of the NaK to ANL-W; Greg Hula of DOE-Idaho for his lead role in initiating and overseeing the funding of the project; Greg Bass and Jim Geringer of DOE-Chicago/Argonne Group West for their lead roles in interfacing with DOE-ID and the State of Idaho; Pat Kern of ANL-W as Facility Supervisor and responsible for the operation of the NaK treatment process at SCMS; Spence Barker of ANL-W for developing and performing requisite chemical analyses; and Roy Grant of ANL-W for effecting the shipment of treated waste to RWMC.
REFERENCES