SOL-GEL TECHNOLOGY AND THE NUCLEAR INDUSTRY
B.J.J. Zelinski, J. Young, K. Davidson, A. Aruchamy, and D.R. Uhlmann
Department of Materials Science and Engineering
University of Arizona, Tucson, AZ 85721
G.L. Smith and H.D. Smith
Pacific Northwest National Laboratory
Richland, WA 99352
ABSTRACT
The unique capabilities associated with sol-gel technology have been explored by the nuclear industry for use in a broad range of applications which include the fabrication of microspheres of U, Th, and Pu oxides for fuel cells, glassy matrices for immobilization of nuclear waste, and present efforts to stabilize radioactive salt wastes. Among attractive aspects of sol-gel technology are the abilities to prepare ceramic powders with tailored particle sizes without generating grinding debris; glasses with novel compositions and properties which cannot be made by conventional means; ceramic bodies with novel microstructures and tailored distributions of phases, including porosity; materials with mixed organic-inorganic (polymer-ceramic) functionality, having unique combinations of properties; chemically homogeneous glass precursors using firing temperatures much lower than conventionally derived materials; and gel structures which can immobilize (temporarily - if desired) waste components in preparation for disposal. This paper provides a description of sol-gel technology and its implementation and capabilities relevant to the nuclear industry. Emphasis is placed on applications of sol-gel technology in the DOE complex and on current work developing sol-gel approaches to stabilize salt wastes contaminated with organic solvents, heavy metals, and/or transuranic elements. The salt component of these wastes typically contains chloride, hydroxide, nitrate, and/or sulfate salts of sodium. Current efforts to stabilize these wastes, which include grout mixing and vitrification schemes, are complicated by the soluble nature of these salts and by the diversity of the contaminants which they contain. Sol-gel technology shows great promise for overcoming these difficulties and facilitating the fabrication of suitable waste forms with high levels of salt loading.
INTRODUCTION
Sol-gel processing involves the dissolution of selected precursor chemicals in an appropriate solvent, and reaction at or near room temperature to form an oxide or organically modified oxide network in the solvent [1-2]. As condensation proceeds in the precursor solution, the formation of metal-oxygen-metal bonds takes place until the oxide-based network either precipitates as a separate phase or extends continuously throughout the solvent. In the latter case, the viscosity of the system rapidly increases and the mixture gels. The gel consists of two interpenetrating phases, one the solid network phase and the other the liquid solvent-rich phase. Reaction conditions such as the type and concentration of solvent, precursor chemicals, and catalyst, rates of addition, and solution pH and temperature determine whether precipitation or gelation will occur. In the case of precipitation, careful control of reaction and drying conditions can produce powders having tailored particle characteristics including shape, size and size distribution. This capability has been exploited in the past by the nuclear industry to fabricate microspheres of U, Th, and Pu oxides for fuel cell applications.
Gelation of the precursor solution drastically reduces the rates of chemical mixing by either diffusion or convection in the liquid phase and imparts mechanical stability to the gelled mass. These characteristics can be exploited in decontamination and decommissioning activities by infiltrating the fluid precursor liquid into regions of contaminated pipes, tanks, etc, which are otherwise difficult to access. Gelation of the liquid will then immobilize dust and debris and increase the mechanical integrity of structurally compromised components. Potential applications include in situ stabilization of tank wastes, spent nuclear fuel elements during removal and transport, and sludges containing high concentrations of spent fuel and radiochemical wastes.
During sol-gel processing, crosslinking reactions create a structural skeleton, the network, which entraps solvent. Weakly crosslinked networks collapse and flow in response to the capillary stresses induced during solvent removal and form dried structures which contain little or no internal porosity. Precursor solutions containing these partially reacted species can be sprayed onto the outer surfaces of contaminated components to form thin films which reduce exfoliation of particulate debris and increase corrosion resistance.
More strongly crosslinked, strong skeletal structures resist drying stresses and form an interconnected fine pore structure of high surface area as the solvent evaporates. In most cases, drying occurs at room temperature; but heating between 100 - 150 C significantly increases the drying rate. Typical pore structures in dried gels are characterized by sizes in the range of 1-5 nm and surface areas as large as several hundred m2/gm. By carefully controlling the reaction conditions, pore structures can be tailored with specific sizes and size distributions. Such pore structures, characteristic of both dried powders and gels, can be used in remediation efforts as filters and selective absorbers.
In most cases, sol-gel processing produces amorphous or glassy materials. The random occurrence of crosslinking reactions in the solvent at room temperature tends to inhibit the generation of crystalline structure in the network. When heated near the glass transition temperature, the highly porous gels and powders flow to reduce surface area and eliminate porosity. This causes densification and consolidation of the sample to form a solid glass. The advantage of using sol-gel processing to fabricate glass is that the densification process occurs at temperatures which are several hundreds of degrees lower than those required to melt glass in more conventional vitrification operations. Consequently, vitrification schemes for immobilization and encapsulation of radioactive wastes can be completed at lower temperatures, thus minimizing the loss of highly volatile components of the waste stream.
Alternatively, the composition and heating schedule of the sol-gel material can be adjusted so that firing causes the amorphous structure to crystallize. In this way, the entrained waste components can be immobilized in a crystalline matrix, which in some cases is more resistant to water attack than the amorphous parent phase. Like vitrification, this glass-to-ceramic processing can be accomplished at temperatures which are much lower than those required to form the ceramic using conventional processing.
In most oxide applications, gels containing SiO2 are utilized, but gels with other compositions can be fabricated merely by changing the precursor chemicals. Gels of SiO2-based materials can be fabricated over a wide range of chemical conditions including high water content and low and high pHs [3]. This flexibility is particularly important for D&D activities, since targeted waste streams in the DOE inventory possess greatly varying chemical and physical characteristics. Also, the use of SiO2 based gels is attractive because SiO2 is a major component in typical waste vitrification schemes and because crosslinking in these types of gels appears to be unaffected by the high radiation environments which may be present during use[4].
TYPES OF GELS
In addition to the previously described forms in which sol-gel derived materials can be obtained, gels for application in waste remediation and removal can be categorized as follows:
POTENTIAL APPLICATIONS
Sol-gel processing techniques offer potential solutions to many of the most pressing environmental restoration problems facing the DOE nuclear complex. This section provides examples which demonstrate the utility of this technology[12]. The next section discusses current research being conducted by the authors to stabilize salt wastes using sol-gel techniques.
In-Canister Encapsulation of Spent Nuclear Fuel Elements
Irradiated Hanford production reactor fuel element assemblies are currently housed in water storage basins. These fuels are stored in sealed or open canisters which sit in racks on the basin floors. Handling damage and corrosion processes have reduced the mechanical strength of many fuel elements. This potentially complicates handling operations, as the corroded fuel elements may lack the strength required to ensure their integrity.
In-canister encapsulation by sol-gel processing could be used to stabilize the corroded fuel elements and encase debris which has settled in the canister bottoms. Implementation could consist of fabricating a gel matrix in-basin which will surround, contain and stabilize the fuel and waste components. The gels could be designed to possess the appropriate mechanical and chemical structure to facilitate remediation, interim storage, and final disposition. As glass/ceramic precursors, these gel systems are compatible with vitrification and similar disposition pathways. In principle, the gel systems are also compatible with low temperature passivation schemes.
Such a system could be employed by delivering a gelling medium to the canisters and inducing gelation, encasing the canisters in the gel and providing mechanical strength to the entire body. The current in-basin crane system may be used to lift the gel-encased canisters into appropriate containers for transportation to a remediation facility. Following canister removal from the basin, the fuel may be separated from the canister and from the sludge and gel components by washing, a reversible gel process, or other means, allowing for different remediation and disposition pathways for the fuel, canister and sludge components. The gel may also be designed to passivate the fuel elements for preprocessing storage.
Stabilization of Fuel Basin Sludge
In some fuel cell storage basins (K-East basin), reaction products from the corrosion and disintegration of fuel elements and other debris have formed a sludge layer on the floor of the basins. Irradiated fuel has accumulated on both the 105-KE and 105-N Basin floors from fuel oxidation and fuel handling operations. In addition to the U and Zr oxides, aluminum oxide/hydroxide, iron oxide, concrete grit, lost tools, miscellaneous hardware, and other oxides and materials are components of the basin sludge. The estimated sludge volumes in 105-KE Basin and 105-N Basin are 2300 and 332 cubic feet, respectively.
Specific problems resulting from particulate resuspension include: turbidity, contamination of basin water quality systems with transuranic wastes and the resulting increase in secondary waste generation, elevated radionuclide concentrations in the basin water and resulting worker exposure issues, and an increased risk of environmental release via airborne pathways.
Sludge stabilization via sol-gel processing is based on the use of wet chemical means to create a gel matrix in-basin which will contain and stabilize the sludge components. In use, the gelling medium will be delivered to the sludge in-basin. Once the gel components have permeated the sludge volume, gelation will be induced, causing the sludge to be incorporated in a gel matrix. The gel system could be designed so that the resulting material has a consistency ranging from a pumpable slurry to a rigid solid, depending upon the desired retrieval mechanism. For a pumpable slurry, the sludge/gel system could be vacuum pumped from the basin, allowing for simplified solid/liquid separation and a much lower degree of sludge resuspension.
D&D of Contaminated Pipe Networks
Facilities used in defense-related nuclear materials development often contain networks of contaminated piping which have been exposed to aqueous environments for long periods of time. In some cases the piping has corroded to the extent that its mechanical integrity is in question; and sectioning and removal of the pipe pose an increased risk of further contamination and airborne radioactivity.
The Hanford N-Reactor Fission Product Trap (105-N) is an example of this type of facility. It was designed as a low pressure flush line for reactor core coolant in the event of fuel breach. Other facilities were provided adjacent to the Trap for removal of corrosion and fission products. Removal of the trap network has been proposed as part of the N-Reactor D&D operations. The steel pipe has been exposed to an aqueous environment likely compromising its integrity. Recent radiological surveys indicate that high levels of contamination and airborne radioactivity exist. No baseline technology has been identified to address such issues.
The use of sol-gel processing for D&D pipe activities is based on the ability to form a solid gel from liquid precursors. This provides the potential to 1) immobilize liquid and surface contaminants inside the pipes, 2) reduce the risk of further airborne contamination during pipe sectioning and removal, and 3) enhance the pipe mechanical strength for handling and transportation.
Such an approach could be implemented as follows: 1) delivery of the precursor solution to fill the inside of the pipe network; 2) gelling to encapsulate the fission and corrosion products in the gel matrix; 3) cutting and removal of pipe sections, during which the gel would stabilize internal surface contamination and minimize airborne contamination; 4) gel removal by washing or by chemically inducing a reversal of gel formation such that the gel materials (which contain encapsulated fission and corrosion products) may be easily separated from the pipe segments; and 5) final disposition of the gel using established vitrification technology and disposal of pipe as low-level waste.
In Situ
Barrier Formation Between Nuclear Facilities or Contamination and the EnvironmentThe initial inherent fluidity of sol-gel systems and the ability to preprogram their gelation rate makes them excellent candidates for the production of engineered barriers to isolate environmental hazards. They can be injected into porous media (e.g., soil) which surround waste tanks, forming a second, independent containment barrier for aging waste tanks. Similarly, sol-gel systems can be used to stabilize trench wastes until final disposition can be accomplished; or such a treatment could provide a permanent solution. This would be accomplished by injecting the gel system into the waste, thus isolating the waste to prevent ground water leaching and preventing wind erosion or animal and human intrusion. Sol-gel materials which harden in the presence of water or under water would be prime candidates for redirecting or sealing off aquifers which might leach buried wastes. They thus offer an alternative to the underground ice dams proposed to protect the Columbia River in the Hanford 100 area.
Containment of Technetium and Other High Temperature Volatile Radionuclides
The disposition pathway of technetium and other radiochemicals which become volatile at or near vitrification melt temperatures remains an open technical issue which must be resolved prior to treating certain defense-related waste streams. At the Hanford site, e.g., low level waste (LLW) and high level waste (HLW) streams contain Tc well beyond the amount which can be disposed of on site, thus requiring the incorporation of the majority of the Tc inventory into HLW form for final disposition. The Tc is present in a variety of forms including in-tank precipitated salts, contaminated ion exchange resins, dissolved species in original waste liquids, and condensation products from offgas vitrification flues. Retaining Tc is difficult because it evaporates rapidly at temperatures required to fabricate conventional HLW forms.
When sol-gel techniques are used to fabricate glasses, the glass network begins forming by chemical reactions in the solution at room temperature. The raw materials are mixed in the liquid state, thus assuring excellent chemical homogeneity. Temperatures lower by hundreds of degrees can be used to fabricate glasses using sol-gel techniques compared to conventional melting approaches. As an example, the low temperature sintering behavior of borosilicate-based glasses can be used to fabricate fully dense glasses with good chemical durability, producing Tc containing HLW forms while significantly reducing evaporative losses of Tc and other volatile radiochemicals.
SOL-GEL STABILIZATION OF SALT WASTES
Salt wastes are typically chloride, hydroxide, nitrate, and/or sulfate salts of sodium which have been contaminated with a combination of organic solvents, heavy metals, and/or transuranic (TRU) elements. Their annual production volumes typically range from 1 m3 to 100 m3, with the exception of the Rocky Flats site (3000 m3). They result from a variety of vitrification and chemical processing operations and are found at several DOE sites, especially Hanford.
The difficulties in dealing with salt wastes result from their high solubility and the diverse nature of the contaminants. They are not good hosts for containing contaminants under moist conditions; and the physical and chemical characteristics of organic solvents are very different from those of the heavy metal or TRU salt compounds. Grouting the waste salts addresses the problems by physically diluting the waste to the extent that it has minimal effect on the physical properties of the host grout and providing a long diffusion (or percolation) path for the escape of the hazardous components; but grouting greatly increases the waste volumes for the final storage facility because salt contents must be limited to 10% or less in order to maintain grout integrity. Traditional vitrification technologies, on the other hand, suffer from the tendency to form secondary waste streams (e.g., salt wastes) due to volatilization at typical vitrification temperatures.
Through a collaborative program between the Arizona Materials Laboratory at the University of Arizona and Pacific Northwest National Laboratory, sol-gel derived alternatives to grouting and melt vitrification for salt stabilization are being developed. The objective is to develop low temperature processing routes with the ability to accommodate high salt loadings (>10% by weight of salt), which use simple processes, minimize the formation of secondary waste forms, and can be implemented safely while still maintaining radionuclide and toxic metal containment which satisfy the NRC Waste Form Stability Requirements.
Towards this end, a series of polyceram gels matrices have been fabricated into which 50 wt% or more salt waste surrogate has been incorporated. Polycerams are materials with network structures which combine carbon or silicon based organic polymers with oxide based ceramics. In most polycerams, the linkage between polymer and oxide components is made through siloxane bonds. Polymers are flexible, deformable, tough, permeable to water, and can be processed using low temperatures. Ceramics are hard, strong, radiation resistant, leach resistant, and water impermeable, but require high temperatures to process. The properties of polycerams lie between those of ceramics and polymers.
Polyceram samples ranging in composition from 100% ceramic to 100% polymeric have been fabricated. The basic characteristics of the waste forms have been qualitatively assessed and the relative chemical durability has been evaluated. Chemical durability was evaluated by determining the concentration of chromium which leached from samples soaked in water of neutral pH at room temperature for 6 weeks. Being highly soluble in water, the chromium oxide in the waste surrogate will behave in a manner similar to that of the salts. Its corrosion or leaching will be similar to that of the salt component of the waste form. Since the other toxic elements are largely water insoluble at neutral pH, they will likely be more easily retained in the waste form than is chromium. Thus, the results from leaching studies of chromium should represent the worst case for leaching of the toxic metal components.
The chromium concentration of the supernatant was determined using a Jobin-Yvon Spex fluorescence spectrometer model FluoroMax-2. The absorption line of chromium at 285 nm was selected for excitation and the emission spectrum was measured in the 300-700 nm range. The intense chromium emission peak at 575 nm was used to determine chromium concentration. Samples of supernatant were measured in the spectrometer and compared to standards prepared using distilled water and CrO3.
The following section describes each of six different sol-gel derived materials which were investigated for their potential use as salt stabilization matrices. The advantages and disadvantages of each case are discussed in turn after which their chemical durabilities are compared. Finally, two routes are identified for further development work.
Ceramic Matrices
Two approaches were taken to fabricate 100% ceramic matrices for stabilizing salt wastes. First, the sol-gel chemistry was chosen so that at elevated temperatures the salt components in the waste surrogate would decompose and become incorporated into the ceramic matrix. The salt waste was added to a sol-gel solution containing precursors to form a sodium aluminum borosilicate glass of composition 58% SiO2, 34% B2O3, 6% NaO, and 2% Al2O3 by weight. The sol-gel solution was formed by mixing partially hydrolyzed Si(OC2H5)4 in ethanol with H3BO3, NaOH, and Al(OC4H9)2(C6H9O3); and 50 - 70 wt% waste was added and stirred until the mixture dried. The sample was pressed into pellets and fired at 800 C for 30 min. to produce a dense glass with good mechanical strength and chemical durability.
This route offers many advantages for salt waste stabilization. The resulting waste form does not contain salts since they have decomposed at the firing temperatures. In essence, the salt waste and sol-gel precursors are converted to an oxide glass; and the waste form is much more durable, harder, and stronger than any forms which incorporate polymers. The disadvantage associated with this technique is the use of firing temperatures about 800 C, although such temperatures are still much lower than the 1100 - 1400 C required for conventional vitrification processing.
The second ceramic approach to salt waste stabilization encapsulates the waste in a ceramic matrix which can be consolidated at relatively low temperatures. In this approach, the salt particles were added to a low melting oxyfluoride glass. Tin fluorophosphate glass was used as a model system to test the concept of encapsulation by a ceramic/glass matrix. The glass had a composition of 40:30:30 (by wt) of SnO:SnF2:P2O5. The glass was fabricated by melting SnO2, SnF4 and (NH4)H2PO4 at 550 C. After the glass was cooled, the salt waste was added and the mixture was reheated. At about 300 C, the glass softened sufficiently so that the waste particles began to become encapsulated. Unfortunately, when the temperature was raised to lower the glass viscosity and encourage encapsulation, salt decomposition ruined the sample. To develop further this approach, additional work is needed to modify the glass composition to promote flow at lower temperatures.
The advantages associated with this approach are that the tin fluorophosphate glass is strong, hard, and reasonably durable. High waste loading, largely independent of waste type, can be accommodated because the waste is not decomposed, but instead is surrounded and encapsulated. Mechanical mixing at elevated temperatures may be required to ensure a uniform distribution of the salt waste throughout the waste form, but this could be avoided using sol-gel routes to synthesize the glass.
Polymer Matrices
To evaluate the prospects of using polymers (with no addition of ceramic component via sol-gel techniques), a model system based on the use of polystyrene was investigated. In this approach, polystyrene was dissolved in acetone until the polymer formed a dough-like material. The salt waste surrogate was added at a concentration of 50 wt% by kneading it into the softened polymer. The mixture dried at 60 C until hard. Preliminary results indicate that the salt waste surrogate was encapsulated and uniformly distributed throughout the waste form, which had good mechanical strength and toughness.
The advantages associated with this approach are that it is a simple process which can easily be scaled up using conventional mixing equipment and that it requires no high temperature processing. The disadvantages of this particular system are largely associated with the choice of polymer. Polystyrene is non-polar and thus is not chemically compatible with the surfaces of the polar salt particles. Hence no strong bonding exists between the polymer and the salt phases, which may have a detrimental effect on chemical durability. Also, polystyrene is a relatively weak polymer, but has sufficient strength to meet the NRC compressive strength requirements.
Future work will focus on using polyurethanes in place of polystyrene. Polyurethanes possess excellent strength, durability, and toughness, and are much more polar than polystyrene. The increased polarity facilitates mixing with the salt, reduces phase separation between polymer and salt phases, and increases chemical durability. Regardless of the polymer selected, solvent management and disposal issues are concerns.
Polyceram Matrices
MPEOU-Based Polycerams
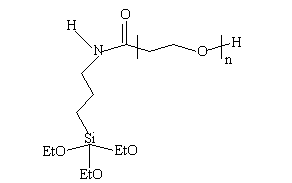
To begin exploration of sol-gel routes to fabricate polycerams for salt waste stabilization, a functionalized polymer was combined with TEOS [Si(OC2H5)4] to form the matrix for encapsulation. The polymer was N-triethoxysilyl propyl O-polyethylene oxide urethane (MPEOU) - Si(OC2H5)3NHCO[CH2CH2O]n-H, whose structure is shown below:

Fig. 1. The structure of MPEOU
The Si atom in the group which extends from the urethane portion of the molecule is bonded to three ethoxy (EtO) groups. The ethoxy groups can be hydrolyzed and condensed to create oxide linkages with the TEOS. A polyceram waste form based on MPEOU was synthesized using a 1:1 ratio of partially hydrolyzed TEOS and MPEOU in a mutual solvent. To this mixture was added 70 wt% of the waste, and the mixture was hardened and dried at room temperature. The resulting waste form had good mechanical properties and contained a uniform dispersion of the waste in the polyceram matrix.
The advantages of this approach are that the polar groups in the polyceram promote bonding to the salt particles, and all processing is carried out at room temperature, where chemical reactions lead to polymer-polymer and polymer-oxide crosslinking. The oxide component of the polyceram improves the strength and leach resistance of the polymer.
As fabricated, the MPEOU polyceram demonstrated considerable potential. It is likely, however, that the low molecular weight of the MPEOU used here compromised the crosslink density and water permeability of the organic component. Future work will focus on the use of functionalized, high molecular weight polyurethanes.
PDMS/Z-6040 Silane Based Polyceram
The second polyceram used OH-terminated polydimethylsiloxane (PDMS), whose formula is shown below
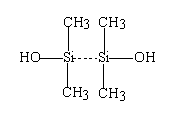
Fig. 2. The structure of PDMS
The backbone of this polymer consists of Si-O-Si bonds; and the SiOH end groups can participate in crosslinking reactions. To promote mixing and bonding between the PDMS and the surfaces of the salt particles, an epoxy functionalized silane, Z-6040 (Dow Corning), was employed.
In detail, Z-6040 was partially hydrolyzed and added to the waste in a 1:1 weight ratio. The treated waste was allowed to dry and then ground into a powder. Partially hydrolyzed TEOS was mixed with PDMS in the weight ratio of 40:60 and refluxed; and the mixture was added to the dried salt waste/Z-6040 mixture, stirred, allowed to set and dried at 78 C. The resulting waste form contained a good dispersion of salt waste and had good mechanical integrity.
In small quantities, the Z-6040 silane promotes bonding between PDMS and the inorganic salt components. In larger quantities, it allows crosslinking between epoxy groups upon heating. Since unmodified PDMS is water permeable, future work will focus on using modified PDMS with functionalized side groups to promote crosslinking and reduce water permeability.
PBD Based Polycerams
The final polyceram studied uses polybutadiene (PBD) as the polymer component. Initial work has utilized an end-functionalized PBD molecule:
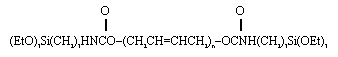
Both ends are terminated by triethoxysilane groups which can participate in crosslinking reactions. The polyceram waste forms were fabricated by partially hydrolyzing TEOS and mixing it with the end-modified PBD to produce a polyceram containing 78 wt% PBD and 22 wt% SiO2. To this mixture was added 50 wt% salt waste. The final mixture was set and dry at room temperature. The waste form was rubbery in consistency but retained good mechanical integrity.
The advantages associated with this route are that PBD is a strong, durable, and tough polymer. It is quite impermeable to water, and should have good chemical durability. Sulfur can be added to increase polymer-polymer bonding to improve further the density, strength and durability. The disadvantage of using the end-modified PBD is that, without the use of sulfur, only the ends of the large PBD molecules can participate in crosslinking reactions. This minimizes the opportunity for crosslinking along the length of the PBD chain
Chemical Durability
Results of the chemical durability of six samples measured by fluorescence spectroscopy are shown below in Table III.
Table I Chemical Durability
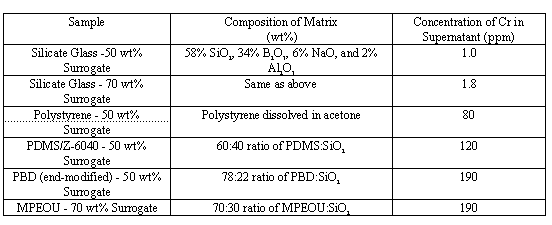
The results indicate that sol-gel derived ceramic matrices provide excellent chemical stability. Immersion of the sample in water for 6 weeks has produced only 1 ppm of Cr in the supernatant solution of the Na-Al borosilicate glass with a waste loading of 50 wt%. When the waste loading is 70 wt%, the leach rate is still very low, as indicated by a Cr concentration of only 1.8 ppm in the supernatant. The supernatant of the polymer and polyceram waste forms contained 80 - 190 ppm of Cr. While these concentrations are much higher than those with the all-ceramic sample, they are not surprising as the oxide glass matrix chemically incorporates the salt ions into its lattice. The polymer and polyceram routes encapsulate the waste, an approach which will work if the water permeability of the polymer matrix can be sufficiently reduced. The polymer based system which exhibited the best corrosion resistance was the polystyrene route. This demonstrates the importance of achieving high crosslink densities and low solvent contents in the polymer component of the polycerams. As the crosslink density in the polycerams is increased and the solvent content reduced, their chemical durability should improve dramatically and exceed that of the polystyrene based system. Polycerams based on side-modified PBD as the polymer component are being fabricated and evaluated. The structure of side-modified PBD is shown below:
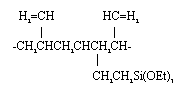
Side groups are attached along the polymer chain which contain functional groups of triethoxysilane. These provide a means to crosslink the PBD molecules, one to another, all along the length of the chain to decrease water permeability and improve chemical durability.
SUMMARY AND CONCLUSIONS
Sol-gel processing is a low temperature technique by which precursors are mixed and reacted to create oxide and organically modified oxide networks in the form of powders, gels, dense glasses, ceramics, and films. The gels are largely insensitive to radiation effects, and most sol-gel materials of present interest are compatible with melt vitrification. Various gels including SiO2 based gels, high solids-content gels, collapsible and reversible gels, light elements gels, and polyceram gels have great potential for nuclear environmental restoration. Applications include in-canister encapsulation of spent nuclear fuel elements, stabilization of fuel basin sludge, decommissioning and decontamination of pipe networks, formation of in situ barriers between nuclear facilities or storage ponds/trenches and the surrounding environment, and stabilization of Tc and other volatile radionuclides. Sol-gel processing is attractive for solving these problems because of its capability of fabricating uniformly sized particles and pores, dense glasses of novel compositions at reduced temperatures, new ceramics with novel microstructures, immobilizing gels, impermeable and corrosion resistant coatings, homogeneous and pure oxides, and organically modified oxides (polycerams). Collaborative efforts between the Arizona Materials Laboratory at the University of Arizona and Pacific Northwest National Laboratories are focused on employing sol-gel methods to stabilize salt wastes which contain organic solvents, toxic metals, and some transuranic elements. Preliminary studies have demonstrated the efficacy of several ceramic, polymer, and polyceram systems. Future efforts will be directed towards developing side-modified PBD polycerams waste forms, which offer outstanding promise for delivering high salt loadings, good mechanical strength and toughness, and excellent chemical durability.
REFERENCES