CHELATING ION-EXCHANGE RESINS FOR THE COMPETITIVE
SORPTION OF LANTHANUM AND EUROPIUM
M. Draye, K. R. Czerwinski
Massachusetts Institute of Technology, Department of Nuclear Engineering
77 Massachusetts Avenue, Cambridge MA 02139, USA
A. Favre-Réguillon, J. Foos, A. Guy
CNAM, Laboratoire des Sciences Nucléaires, 2 rue Conté, 75003 Paris, France
ABSTRACT
The selective removal of europium (homolog of a trivalent actinide) by phenolic ion-exchange resins from aqueous solutions is studied to develop ion-specific resins for the nuclear waste treatment. The resins are synthesized by alkaline polycondensation of formaldehyde with phenol, catechol, resorcinol and 8-hydroxyquinoline. The resins are characterized by the moisture regain, ion exchange capacity (H
+ - >Na+), IR spectroscopy and Europium distribution coefficient (D). Their ionoselectivities for europium and lanthanum are evaluated by solid-liquid extraction and their ion-exchange capacities are compared. The incorporation of 8-hydroxyquinoline in the molecular matrix of the phenolic resins is shown to exert a significant influence upon the competitive sorption of lanthanum and europium, leading to their intragroup separation. The selective (S = 6.2, 6.9, 9.9 ' 5%) sorption of Eu indicates ion selective resins can be prepared for the specific separation of actinide ions from the nuclear waste.INTRODUCTION
The use of nuclear fission reactors in the next several decades will require methods for safe disposal of highly radioactive wastes. One of the most critical aspects of this waste-disposal problem concerns safe long-term storage of the wastes. Society and environment need protection from contamination by the long-lived transplutonium elements. The waste products contain a variety of radioactive elements, including long-lived
a emitting actinides (An) which require an extended period of storage. The An storage problem could be reduced by separating the a -emitting species from the fission products and storing them separately. The main obstacle in the separation of the transplutonium elements from other fission products is the separation of the An from the lanthanides (Ln), which have similar ionic radii (Table I) [1] and electronic structures.Table I. Ionic Radii of 6 Coordinate Trivalent Lanthanide Ions, Am3+, and Cm3+
|
Element |
Radius (Å) |
Element |
Radius (Å) |
Element |
Radius(Å) |
|
La |
1.03 |
Sm |
0.958 |
Ho |
0.901 |
|
Ce |
1.01 |
Eu |
0.947 |
Er |
0.890 |
|
Pr |
0.99 |
Gd |
0.938 |
Tm |
0.880 |
|
Nd |
0.983 |
Tb |
0.923 |
Yb |
0.868 |
|
Pm |
0.97 |
Dy |
0.912 |
Lu |
0.861 |
|
Am |
0.975 |
Cm |
0.97 |
|
|
Numerous methods based on liquid-liquid extraction[2,3] or solid-liquid extraction[4,5] have been described for effecting this separation[6,7]. Among them, solid-liquid extraction is considered more effective. From the waste management point of view, the most attractive feature of this technique is its ability to partition radioactive elements into two separate components yielding a small volume of decontaminated effluent suitable for direct disposal. Many studies have pointed out the use of mineral matrix[8] constitutes additional waste. However, organic resins, containing only C, H, O, N atoms, are completely incinerable. Ion exchange resins containing phenolic (
- OH) groups have been known to possess exceptionally high affinity to Cs and Sr [9-12]. In this article the synthesis and uptake properties are reported for Eu3+ of resins obtained by alkaline polycondensation of formaldehyde with catechol and resorcinol. Introduction of 8-hydroxyquinoline in the molecular matrix is shown to induce an intragroup separation of lanthanum and europium.EXPERIMENTAL
Materials
Phenol, resorcinol, catechol, 8-hydroxyquinoline and formaldehyde (37% aqueous solution) were obtained from Aldrich and used without further purification. The europium salts used were europium nitrate pentahydrate and hexahydrate from Aldrich. The lanthanum salt used was lanthanum chloride heptahydrate from Fluka.
FTIR Spectroscopy
FTIR spectra were recorded on a 1600 Perkin-Elmer spectrophotometer. The measurements were done using KBr pellets in the range 4000-400 cm
-1.Organic Synthesis
The general method of Pennington and Williams [12] was followed with some modifications previously described [9]. The resins were synthesized by alkaline polycondensation of formaldehyde with phenolic compounds.
General synthesis of the phenolic resins
. A 11.01 g sample of phenolic compound (0.1 mol) was dissolved in 75 mL of 2N sodium hydroxide (0.15 mol). A 19 mL sample of 37% formaldehyde (0.25 mol) was added (phenolic compound/formaldehyde molar ratio of 1:2.5). After adding the reactants, the mixture was stirred and left overnight. The mixture was then kept in an air oven at 100°C to cure for four days. After curing, the product black polymer was crushed, sieved to 63 to 250 m m size particles, washed, and conditioned by subjecting it to two 1N NaOH / 1N HCl cycles with a water wash in between. The resin is finally converted to the H+ form and washed thoroughly with water until neutral. The resin was then air-dried (60°C).Catechol-formaldehyde resin (CF). This was prepared from catechol. FTIR, (KBr): 3280 cm
-1 : n OH bonded; 2924 cm-1 : nasCH2; 1600, 1578, 1525, 1438 cm-1 : n C=C aromatic; 1283, 1174 cm-1 : n =C-O and d OHResorcinol-formaldehyde resin (RF). This was prepared from resorcinol. Before addition of the formaldehyde, the mixture was cooled to 0
°C and, formaldehyde was rapidly added leading to a temperature of 0°C ± 0.2°C.FTIR, (KBr): 3406 cm
-1 : n OH bonded; 2933 cm-1 : n asCH2; 1618, 1474, 1561, 1439 cm-1 : n C=C aromatic; 1294, 1205 cm-1 : n =C-O and d OHGeneral synthesis of the phenolic-8-hydroxyquinoline resins
. A thick, yellow slurry was formed by addition of 7.26 g (0.05 mole) 8-hydroxyquinoline to 30 mL of 1.5N sodium hydroxide (0.045 mole, 1.8 g). A 10.14 g sample of 37% formaldehyde (0.125 mole) was added to this and slowly reacted to form a deep red solution. After 15 minutes a solution of 0.05 mole of phenolic compound in 30 mL of 1.5N sodium hydroxide (0.045 mole, 1.8 g) was added, followed by 10.14 g of 37% formaldehyde (0.125 mole). The mixture was then kept in an air oven at 100°C for four days to cure. After curing, the product red polymer was crushed, sieved to 63 to 250 m m size particles, washed, and conditioned by subjecting it to two 1N NaOH / 1N HCl cycles with a water wash in between. The resin is finally converted to the H+ form and washed thoroughly with water until neutral. The resin was then air-dried (60°C).Phenol-8-hydroxyquinoline-formaldehyde resin (PQF). This was prepared from phenol. FTIR, (KBr): 3355.9 cm
-1 : n OH bonded; 2919.2 cm-1 : n asCH2; 2848.7 cm-1 : n aCH2; 1596.3, 1542.9, 1499.7 cm-1 : n C=C pyridine; 1460 cm-1 : n C=C aromatic; 1155.1, 1366.7 cm-1 : n =C-O and d OHCatechol-8-hydroxyquinoline-formaldehyde resin (CQF). This was prepared from catechol. FTIR, (KBr): 3354 cm
-1 : n OH bonded; 3060 cm-1 : n =CH aromatic; 2919 cm-1 : n asCH2; 1600, 1578 cm-1 : n C=C aromatic; 1631, 1496, 1458 cm-1 : n C=C pyridine; 1155, 1367 cm-1 : n =C-O and d OHResorcinol-8-hydroxyquinoline-formaldehyde resin (RQF). This was prepared from resorcinol. FTIR, (KBr): 3360 cm
-1 : n OH bonded; 2919 cm-1 : n asCH2; 2849 cm-1 : n sCH2; 1601, 1578 cm-1 : n C=C aromatic; 1631, 1500, 1455 cm-1 : n C=C pyridine; 1155, 1361 cm-1 : n =C-O and d OHMoisture Regain
This was determined by heating 0.1 g of resin in an air oven at 100
°C for 24 hours, the loss of weight giving the percentage of water in the resin.Ion-Exchange Capacity
For determination of total ion-exchange capacity, 0.25 g of resin in the H
+ form of known moisture regain was equilibrated overnight with 50mL of 0.1N NaOH solution containing 5% NaCl. The amount of NaOH consumed in the H+ - Na+ exchange was determined by titrating the remaining NaOH in the supernatant with 0.1N HCl solution.Distribution Coefficient
As a measure of the resin's affinity for europium and for comparison between various resins, a standard procedure was followed for the measurement of distribution coefficients, D(mL/g
(dry)).H
+ - form resin. A 0.05g sample of H+-form resin of known moisture content was equilibrated 20 hours at room temperature with 10 mL of an aqueous metal ion solution.O
- - form resin. Before extraction, the resin was converted in its O- - form. A 0.05g sample of H+-form resin was shaken for 2 hours with 10 mL of 1N NaOH. The resin was then filtered and washed with water until neutral. The 0.05g of O- - form resin of known moisture content was then equilibrated 20 hours at room temperature with 10 mL of an aqueous solution of metals.The supernatants were removed and the concentration of lanthanum and europium in the solutions was determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) (Spectro D ICP system from Spectro Analytical Instruments). The distribution coefficient D was calculated by using the formula:
![]()
where C
i and Cf are the initial and final concentrations of Eu, V is the volume of the equilibration solution in mL and m is the weight in g of air-dry H+form resin taken.The concentration of sodium released by the resin was determined by ICP-AES.
RESULTS AND DISCUSSION
The five resins were synthesized with phenol[a], resorcinol[b], catechol[b] and 8-hydroxyquinoline[d] (Fig. 1).
Fig. 1. Phenol[a], catechol[b], resorcinol[c] and 8-hydroxyquinoline[d] molecules.
Alkaline polycondensation of formaldehyde with phenolic compound gives an infusible, insoluble, amorphous, crosslinked polymer. By assuming the complete crosslinking, the repeating unit in such a polymer is given by the following expression[9].
where n = 1 for phenol and n = 2 for catechol as well as resorcinol. If all the
- OH groups are accessible in the polymer, the theoretically expected H+ - Na+ ion-exchange capacity in alkaline solution would be 8.9 meq/g for phenol and 15.6 meq/g for the two dihydroxybenzene resins. The results of the characterization of the resins are presented Table II.Table II. Moisture Regain and Ion-Exchange Capacities for Phenolic Resins.
|
Polymer |
Moisture Regain |
Ion-Exchange Capacity |
|
|
CF |
20 |
8.6 |
55 |
|
RF |
40 |
11.5 |
74 |
|
PQF |
10 |
5.9 |
80 |
|
CQF |
20 |
9.6 |
70 |
|
RQF |
19 |
9.9 |
70 |
The ion exchange capacities for the catechol and resorcinol formaldehyde resins are lower than the theoretical capacities but they are higher[10] than and comparable[9] to those of the literature. Comparison of the experimental with the theoretical values shows that 55% of phenolic groups for the CF resin and 74% for the RF resin were occupied. The ion exchange capacities obtained for the resins made from 8-hydroxyquinoline and a phenolic compound are slightly lower than those obtained for the catechol and resorcinol resins.
Europium Sorption
Many ion exchange materials based on phenol-formaldehyde condensation polymers have been described in the literature together with details of their chelating behavior toward metal ions and selectivity[9-12], which is the main feature of chelating ion exchange. Weakly acidic phenolic groups of the resins showed, as expected, a low metal adsorption in the acid range (pH=4), and results obtained for the five resins are presented in Table III.
Table III. Distribution Coefficients D for the phenol-formaldehyde resin
in their H
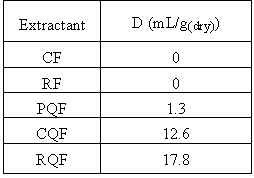
Catechol and resorcinol resins in their H
+ - form are inefficient for europium sorption, whereas 8-hydroxyquinoline formaldehyde resins showed low distribution coefficients. Indeed, ion-exchange sorption of the europium cation is controlled by deprotonation of the phenolic groups of the resin. Phenolic groups are weak acids (pka = 9.9) and are deprotonated at high pH where precipitation of the lanthanides occurs. To avoid precipitation of the Eu and still enhance its sorption, it is then necessary to transform the resins in their Na+ - form before extraction, using the procedure described in the experimental section. Typical batch distribution data for Eu(III) with the five resins are given in Table IV.As a general manner, the efficiency of the resins increases when the europium concentration in the extracted decreases. The introduction of a 8-hydroxyquinoline group in the matrix of the polymers leads to less efficient materials.
Table IV. Distribution Coefficients D and Amount of Eu extracted by substituted phenol-formaldehyde resin in their Na
+ - form, for various concentrations of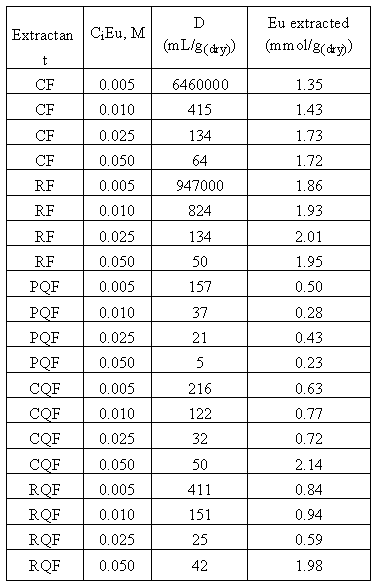
Lanthanum-Europium Competitive Extraction
Separation procedures employing ion exchange resins are frequently made more selective by addition of complexing agents during absorption or elution. Although the most stable and most selective complexes often involve chelating compounds, some of these, because of size and solubility characteristics, are not suitable for the usual ion exchange techniques. To avoid these difficulties and still take advantage of the selectivity of chelating agents, some resins have been synthesized which incorporate the chelating compound in the structure of the resin itself. Resins made from 8-hydroxyquinoline and phenolic compound contain both weakly acidic and weakly basic groups.
Using to the results obtained for Eu extraction, the resins were, before extraction, converted in their Na
+ - form using the procedure that is described in the experimental section. Europium and lanthanum extraction is slow and control experiments showed that a shaking time of 20 hours of contact is required for the europium to produce equilibrium sorption results. Indeed, the rate of Eu3+ sorption of the resin was determined to find the shortest time period for which equilibrium could be carried out while operating as close as possible to equilibrium conditions. The results of the sorption kinetics of europium and lanthanum from a pH 4 solution are shown in Fig. 2.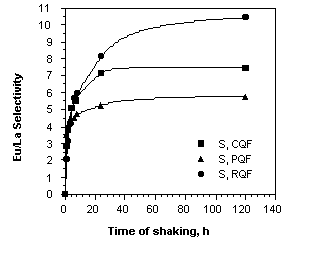
Fig. 2. Europium-lanthanum selectivity profiles for the phenol- catechol- and resorcinol-8-hydroxyquinoline formaldehyde resins as a function of the time.
[La] = [Eu] = 0.0025 mol L
The extent of europium-lanthanum selectivity increases rapidly and appreciably up to 20 hours of shaking, at which time a plateau is reached. So, 20 hours are required for an efficient separation of europium from lanthanum with a considerable selectivity. The RQF resin, which is the most selective, shows a maximum selectivity of 10.4 for the europium in comparison with the lanthanum, after 20 hours of shaking. Results for competitive sorption of lanthanum and europium from aqueous solution are presented in Table V.
Table V. Distribution coefficients D (mL g-1dry) and selectivities for the competitive extraction of Lanthanum and Europium by phenolic resins. Standard deviation ' 5.
[La] = [Eu] = 0.0025 mol L-1, V = 10 mL, pH = 4, m = 0.05 g, T(shaking) = 20 hours.
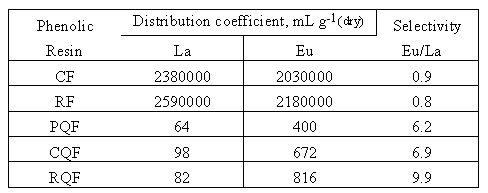
After extraction with the catechol and the resorcinol formaldehyde resins, the concentrations of lanthanum and europium in the aqueous solution are to low to be measured by ICP-AES with a satisfying statistic (standard deviation). Their distribution coefficients are very high but they show a weak selectivity. Incorporation of 8-hydroxyquinoline in the polymeric matrix leads to slightly less efficient resins but induce selectivity between lanthanum and europium. The phenol-, catechol-, and resorcinol-8-hydroxyquinoline- formaldehyde resins stripped Eu with a Eu/La selectivity of 6.2, 6.9 and 9.9 respectively.
These results demonstrate that the sorption process involves both the ion exchange sites of the phenolic compounds and the 8-hydroxyquinoline. The phenolic group provides a cation access site and 8-hydroxyquinoline affords the cation sorption selectivity. The separations are likely based on the small differences in the radii.
CONCLUSION
The results of this study demonstrate that simple phenolic polymers are effective in removing europium from an acidic aqueous medium. The transformation of the resins in cation-exchangers markedly enhanced the europium sorption. The incorporation of 8-hydroxyquinoline into the phenolic resins produces important changes in the competitive sorption of lanthanum and europium. Compared with the resorcinol and catechol formaldehyde resins, the phenol, catechol and resorcinol 8-hydroxyquinoline formaldehyde were slightly less effective but showed a selectivity between lanthanum and europium. These results suggest that by incorporating an appropriate chelating compound in the structure of an efficient ion-exchanger, it will be possible to prepare selective material for trivalent actinide ions.
REFERENCES