SOLVENT EXTRACTION OF CESIUM FROM ALKALINE
WASTE USING CROWN ETHER
Vyacheslav M. Abashkin, Evgeny A. Filippov, Anna K. Nardova,
and Igor V. Mamakin
Russian Research Institute of Chemical Technology
33 Kashirskoe Ave. Moscow 115230
Tel/FAX: (095) 324-8869; E-mail: vm.abas@g23.relcom.ru
Dennis W. Wester
Battelle PNNL
Battelle Boulevard, P/O Box 999
Richland ,WA 99352, USA
ABSTRACT
Experimental results on removal of Cesium-137 from simulated alkaline (NaOH + NaNO3) tank waste demonstrated that proposed solvent extraction system - dibenzo-21-crown-7 (DB-7) solution in special mixture of fluorinated and higher alkylalcohols, has the most promising technological parameters and meets major requirements for efficient reprocessing of alkaline tank waste.
DB-7 extracts cesium from the sodium hydroxide/nitrate solutions lowering with total sodium concentration. Upper acceptable level of Na concentration is about 3.5 M.
The selected extraction system on the base of DB-21-C-7 is proposed for further testing and demonstration in multi-pass counter-flow regime in the modified liquid-liquid extractor ("mixer-settler"), which is now in progress.
The solubility and loss of DB-7 and solvents while dynamic solvent extraction/re-extraction process were estimated. The detailed determination of chemical and radiation stability of two-phase system approved its applicability for reprocessing of real HLW. The irradiation causes high yield of carbonyl adducts due to oxidizing of higher alcohol and fluoride-ion - due to decay of fluorinated alcohol. There is no lost of crown ether after irradiation.
INTRODUCTION
Solvent extraction method is being developed to selectively remove Cesium-137 from radioactive alkaline wastes stored in underground tanks at the U.S. DOE Hanford Site. These wastes were generated during the production of plutonium for use in atomic weapons. Cesium-137, a radioactive long-lived (t1/2 = 30 yr.) radionuclide producing high gamma radiation, is a major concern to DOE and nuclear industry, especially in environmental and waste management of Hanford Site alkaline wastes. These wastes also have high concentration of sodium nitrate and other inorganic salts, which places a premium on the selectivity of any cesium removal process. Macrocyclic polyether Dibenzo-18-crown-7, which exhibits high selectively form complexes with Cs+ , provide good potential for efficient separation of cesium from such solutions.
The present work aims to evaluate crown ethers having high selectivity of cesium over sodium for possible treatment of the simulated Hanford Site tank waste in counter-current regime using the mixer-settler extractor.
EXPERIMENTAL
The DB-7 (98%) was synthesized in the State Research Institute of Chemicals and Super pure Materials (Moscow, Russia). Deionized water was prepared by passing distilled water through an ion-exchange column. Simulated aqueous solutions were prepared in compliance with specifications provided by Battelle PNNL's specialists and used as test solutions.
Organic phase stock solutions of 0.06 M crown ethers were prepared in the mixture of two diluents: 90% of Fluorinated Alcohol (telomer Y-3) and 10% of Isododecylalcohol-IDA (2,4-Diethyloctanol) . This mixture was determined earlier in result of systematic study of different organic diluents and was patented for decontamination of nitric acid solutions.
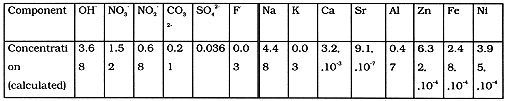
The simulated alkaline waste was prepared - on the base of specifications provided earlier by Battelle and reflecting the approximated chemical composition of Hanford Site (DOE) tank wastes by dissolution of the appropriate salts (mainly - nitrates) in the deionized water, agitation for 4 hours followed by filtration. The composition of the simulated waste is shown in the Table I. A radioactive label isotope 137Cs (4*10-3 mCi/1) was introduced into model solution for radiometric inspection.
Table I. Composition of Simulated Solution, Mole per Liter

The productivity of every cell is in the range from 50 to 150 ml per hour (total flow). Such condition guarantees the most convenient kinetic and thermodynamic parameters for recovery of Cesium also as the most efficient mixing and separation of organic/aqueous flows. The pulsing/injection interface agitation was provided by special drive/distributor with frequency of 50 s-1. The measuring out of organic and aqueous stock solutions was operated by the dosing driver, which assured the continuous and stable operation of the mixer-settler at least for 24 hours. The concentration of Cs was controlled radiometrically, and concomitant elements - spectrophotometrically.
The dose rate, determined using a ferrosulfate dosimeter was 1.7 W*h/1 (1.05*1016 eV/ml*s) taking into account the electronic density of organic solution. Maximum total dose of gamma-irradiation was 80 W*h/1 (1.6*1021 eV/ml). The specimens of irradiated organic solution - DB-7 in the mixture of fluorinated alcohol (FA) and higher alcohol - after phase separation was analyzed to determine the hydrodynamic parameters. Both organic and aqueous phases were analyzed to estimate the decay of extractant and the composition of radiolysis products.
The primary emphasis for the radiolytic experiments was given to the estimation of the FA decay by means of measuring the fluoride-ion concentration in the aqueous phase. It was shown that this value increases through the dose of 60 W*h/1 and then decreases again Such behavior may be attributed to an interaction of the fluoride ion with nitric acid and formation of volatile or organic phase extractable compounds.
RESULTS
Solvent Extraction in Counter-current Regime
The principal flow sheet included totally 20 passes: 8 passes of extraction, 2 passes of washing up, 8-10 passes of back extraction and regeneration of the recycling organic solution (Figure 1). The flow commutation of the mixer-settler's cells was different depending on the used Cs-stripping method. Hydrodynamic testing of the modified MS using the determined earlier organic solution (DB-7 in the mixture of fluorinated and higher alcohols) and stimulated alkaline NaNO3 solution was carried out. There were determined the fixed height of phase separation boundaries, approximate losses of organic solution at different regimes. Special attention was paid to the matching of separate units' operation.
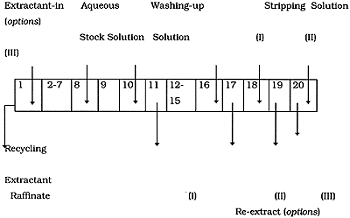
Figure 1. Counter-current Scheme of the Solvent Extraction Process for Decontamination of Alkaline Waste Solution from Cs
To increase the back-extraction efficiency, the re-extraction flow was directed in three different ways. In all options the buffer phosphate solution (0.05 M Na2HPO4 + 0.05M KH2PO4) was used for Cs stripping. Diluted (0.05 M) HNO3 was used to wash up the saturated extract.
Because of very high alkalinity and viscosity of the initial aqueous solution, it was diluted with distilled water (1:1). This procedure provided an improvement of hydrodynamic parameters and phase separation, also as the higher distribution coefficient of Cs, 6.84, in comparison with 3.2 for original stock solution.
The usage of concentrated (8 M) nitric acid solution as a washing-up agent gives low efficiency of Cs back-extraction because of an over-acidulation of the recycling extractant (exp. # 1) in the Table III. Diluted (0.1 M) nitric acid solution (exp. #2) provided efficient extraction and stripping of Cs. In both schemes the flow rates have the following values (ml per hour): extractant - 59.0; aqueous stock solution - 57.33; washing-up solution - 5.66; stripping solution: 15.33.
Table II shows the principal results of reprocessing the diluted (1:1) simulated Cs-containing stock solution, including concentration of Cs and DB-7.
Table II. Equilibrium Concentration of Cs and DB-7 After 10 Hours of Continuous
Operation of the Mixer-settler
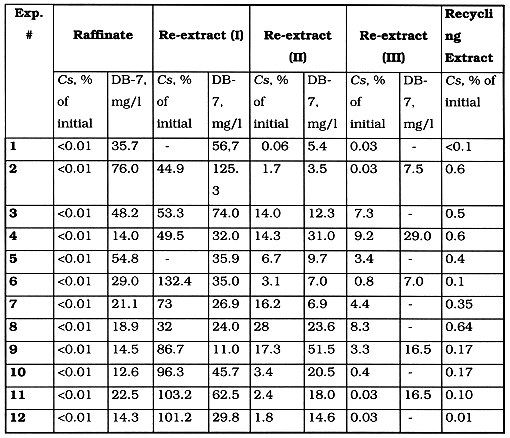
Table III. Dependence of DCs on Concentration of DB-7

There were treated more than 10 1 of simulated waste solution for 6 hours of continuous operation. The achieved cesium concentration degree was up to 2.5. The mean cesium yield as re-extract solution was near 99%. Average cesium dumping, after achieving the stationary regime of extraction cascade, was 5%. Average cesium accumulation in the recycling organic solution was less than 0.3% of initial value.
It was determined that long recycling (5-10 full cycles) of extractant results to gradual decreasing of Dcs down to 2.0, perhaps because of co-extraction of competing elements. Meanwhile the concentration of DB-7 was practically constant for all testing period. Analysis of technological samples from different parts of the extractor has shown that lead , despite of its relatively low concentration in the stock aqueous solution, is accumulated in the recycling organic solution.
Composition of the Extracted Complex
There was determined the Cs: CW ratio in the complex extracted to organic phase, by means of so called "equilibration shift" method. In compliance with this method a number of molecules of complexing agent per one molecule of coordinated ion may be calculated as the tangent of the slope angle of a straight line expressing the bilogarithmic dependence of distribution coefficient versus free concentration of the extractant in organic phase. The data shown in the Table III, allowed to determine the ratio Cs: DB-7 is near 2: 3, corresponding to so called "club-sandwich" structure.
Radiation Stability
Accumulation of Radiolysis Products
The concentration of DB-7 in organic solution on irradiation was measured spectrophotometrically using earlier described technique . Analysis of Infrared Spectra of irradiated extractant in the range since 1500 through 2000 cm-1, has shown a formation of carbonyl derivatives (1730 cm-1), organic nitrates (1640 cm-1) and nitrites (1680 cm-1) produced in result of oxidizing and nitration of the diluent - higher alcohol. Radiation yields for these adducts - measured using appropriate kinetic diagrams (Figures 2 and 3). are: 10.5 (R=CO), 1.3 (R-ONO2), and 1.5 (R=NO2) molecules per 100 eV.
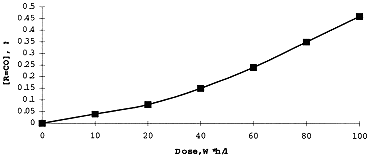
Figure 2. Formation of Carbonyl Adducts While Irradiation of the Extractant
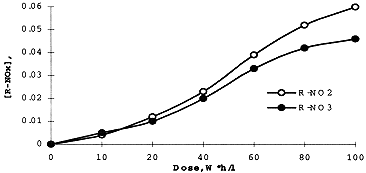
Figure 3. Formation of Nitrate and Nitrite Adducts While Irradiation of the Extractant
Special attention was paid to an accumulation of fluoride-ions in the aqueous phase as a result of radiation decay of the solvent (FA) given in Figure 4. The concentration of F- increases up to a total dose 60 W*h/1 and then slightly decreases. A possible reason for the maximum is the interaction of fluoride-ions with nitric acid and a formation of volatile or extractable compounds. Initial radiation yield of F- is 5.5 ions per 100 eV, while the highest concentration of fluoride is 0.12 g*ion per liter - below the permissible corrosion-proof level of the standard extractor.
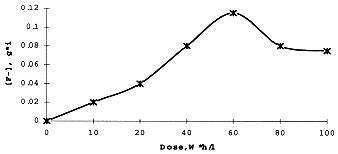
Figure 4. Accumulations of Fluoride-ions in Aqueous Phase as a Result
of Gamma-Irradiation
Concentration of DB-7 on irradiation
During gamma-irradiation the decay of DB-7 and its concentration in organic phase was constantly measured. It was shown (Figure 5) that less than 10% of initial crown ether was lost on irradiation up to 100 W*h/1. It is additional evidence of surprisingly great radiation stability of macrocyclic polyethers.
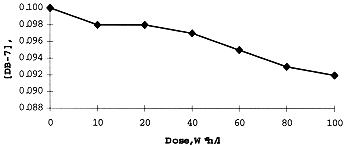
Figure 5. Concentration of DB-7 During Gamma-Irradiation
CONCLUSIONS
Thus, DB-7 in the mixed solvent (FA+IDA) may be recommended for decontamination of Hanford Site tank wastes from 137Cs using counter-current solvent extraction.