THE DATA ON RADIONUCLIDE FORMS IN SOLUTION AS A BASIS FOR DEVELOPMENT OF METHODS FOR LIQUID RADIOACTIVE WASTE TREATMENT
Toropov I.G., Davydov D.Yu., Efremenkov V.M.
Academy of Sciences
ABSTRACT
The sorption behaviour of U(VI), Zr(IV), Pu(IV), Th(IV), Fe(III), Cr(III) with respect to silica gel and cation exchange resin (KU-2) was studied in the present work as a function of their form in aqueous solution. It has been shown that mononuclear hydroxocomlexes of Th(IV) and U(VI) are better sorbed on cation exchange resin than their hydrated complexes. It has been also shown that the sorption of Fe(III), Cr(III) and Pu(IV) on silica gel increases with increase of their mononuclear hydroxocomplexes concentration in solution and sorption of these hydroxocomplexes on silica gel proceeds not through an ion exchange mechnism. An approach to development of technologies for liquid radioactive waste treatment has been suggested.
INTRODUCTION
Purification of liquid radioactive waste is extremely important though rather difficult task. This is because their composition may often be complicated by the presence of different complexing anions, surfactants, besides, the radionuclides in radioactive streams may substantialy differ by their chemical properties. The hydrolysis of radionuclides which may occure in aqueous solution under weak acid, neutral or basic conditions, makes the study of the phisico-chemical processes taking place in such systems even more complicated.
The behaviour of radionuclides in such processes as sorption, ion-exchange, solvent extraction, ultrafiltration, etc., that are used for removal of radionuclides from aqueous solutions, is largely determined by their distribution between various complex forms in solution which, in its turn, depend on pH of solution, concentration of radionuclide and presence of complexing anions. The following species may form in aqueous solution as a result of radionuclide hydrolysis and complexation : hydrated complexes, complexes with complexing anion, mononuclear hydroxocomplexes, polynuclear hydroxocomplexes, particles of colloidal size, besides, at present, there is some evidence of formation of mixed ligands complexes in aqueous solution. Each of the above species has individual physico-chemical properties. Therefore, the more profound our knowledge is about the composition, structure, thermodynamic stability of radionuclide complexes, and the interaction mechanism between each complex form and sorbent, the more efficient one may expect to be the technical solution for the extraction of the radionuclide.
EXPERIMENTAL
The sorption behaviour of U(VI), Zr(IV), Pu(IV), Th(IV), Fe(III), Cr(III) with respect to silica gel and cation exchange resin (KU-2) was studied in the present work as a function of their form in aqueous solution. The content of metal ions was determined using their radioactive isotopes. The results were calculated as coeficients of distribution - Kd, and in the case of Pu(IV) - as percentage of sorption. The distribution of radionuclides by hydroxoforms due to hydrolysis process in a wide range of pH in solution was studied in our previous works [1] applying a set of physico-chemical methods - spectrophotometry, dialysis, ultrafiltration and ion exchange.
RESULTS AND DISCUSSION
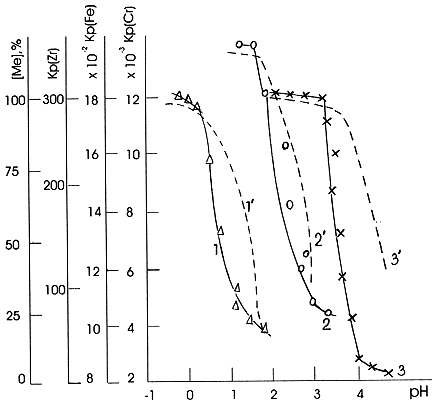
The data presented in Fig.1 show that for Zr(IV), Fe(III) and Cr(III) formation of the mononuclear hydroxocomplexes Me(OH)z-1+ results in a decrease of their sorption on cation exchange resin. In this case normal ion exchange process takes place when a decrease of the cation charge leads to a decrease of its sorption ability. A different behaviour was observed for the sorption of Th(IV) and U(VI). It is seen from the Fig.2. that with growth of the Me(OH)z-1+ hydroxocomplexes concentration in solution the sorption of these metals increases. This testifies to an enhanced sorption ability of hydrolysed Th(OH)3+ and UO2(OH)+ forms with respect to cation exchange resin comparing with that of corresponding hydrated Th4+ and UO22+ forms.

Figure 1. Sorption of radionuclides by cation exchange resin KU-2: 1 - Zr(IV), 2 -Fe(III), 3 -Cr(III); decrease of hydrated metal ion concentration in solution as a result of hydrolysis: 1' - Zr4+, 2' - Fe3+m 3' - Cr3+
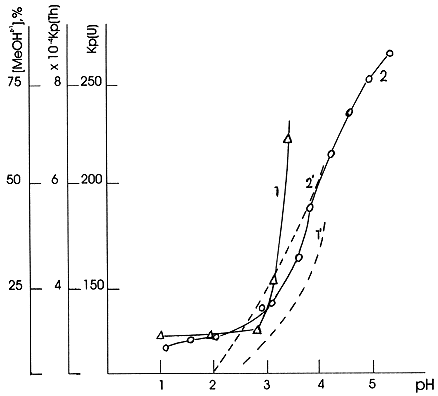
Figure 2. Sorption of radionuclides by cation exchange resin KU-2: 1 - Th(IV), 2 -U(VI); increase of concentration of metal ion mononuclear hydroxocomplex in solution as a result of hydrolysis: 1' -- Th(OH)3+, 2' - UO2(OH)+
Dependance of the radionuclides hydroxocomplexes sorption behaviour on the presence of an inactive electrolyte in solution was investigated in order to determine the nature of their sorption on the cation exchange resin (Fig.4). The results obtained show that the value of distribution coefficient (Kd) decreases with increasing concentration of the competing Na+- cation in solution. This indicates that the sorption of hydrolysed forms of the radionuclides proceeds via an ion exchange sorption mechanism.
According to the data, shown in the Fig.3, the sorption of Fe(III), Cr(III) and Pu(IV) on silica gel increases with increasing concentration of their mononuclear hydroxocomplexes at the constant ionic strength in solution. Thus, the hydroxocomplexes of the above radionuclides are sorbed better on silica gel than their hydrated forms.
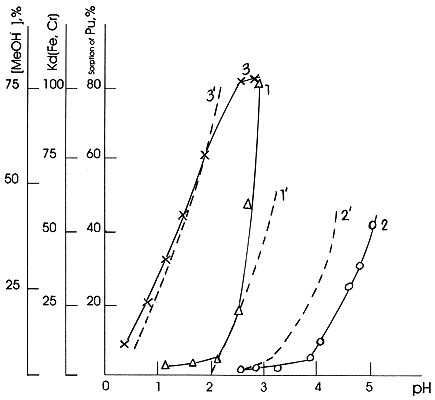
Figure 3. Sorption of radionuclides by silica gel: 1 - Fe(III), 2 - Cr(III), Pu(IV); increase of concentration of metal ion monomuclear hydroxocomplex in solution as a result of hydrolysis: 1' - Fe(OH)2+, 2' - Cr(OH)2+, 3' - Pu(OH)3+
The effect of inactive electrolytes on the sorption of Fe(OH)2+, Cr(OH)2+ and Pu(OH)3+ hydroxocomplexes on silica gel was investigated. It has been shown that the sorption of these radionuclides hydroxocomplexes increases in the presence of Na+, Ca2+ - ions insted of decrease that one might expect if the sorption was of an ion exchange nature (Fig.4). The presence of triply charged aluminium cations in solution of Fe(III) does not affect the value of its distribution coefficient (Kd).
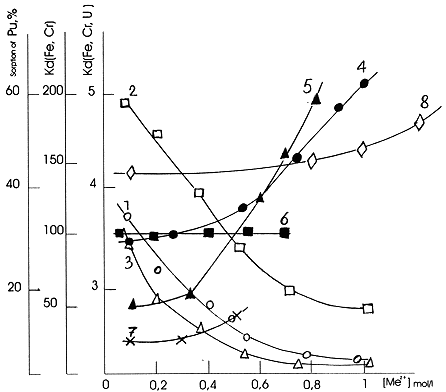
Figure 4. Dependance of the radionuclides sorption on cation exchange resin (1 - 3) and silicagel (4 -8) on concentration of inactive electrolite in solution: 1 - Fe(III), Na+, pH=2.5; 2 - Cr(III), Na+, pH=4.0; 3 - U(VI), Na+, pH=4.5; 4 - Fe(III), Na+, pH=2.8; 5 - Fe(III), Ca2+, pH=2.7; 6 - Fe(III), Al3+, pH=2.7; 7 - Cr(III), Na+, pH=4.5; 8 - Pu(IV), Na+, pH=2.0
The total quantity of OH-groops per gram of silica gel is about 1021 and 1-2 . 1018 of them are dissociated in weakly acid solution. According to our results 1 gram of silica gel sorb approximately 1020 chromium atoms at pH 5.9 and the Cr(III) concentration in solution 5.7 .10-3 mol/l. So, not more then 2% of the sorbed chromium can be distributed among the dissociated Si-O-H groops while the other 98% should occupy the undissotiated SiOH groups. Similar calculations show that only 15% of the iron can be sorbed by the ion exchange mechanism.
All of the above proves that the sorption of the radionuclides proceeds through the mechanism other than ion exchange. In our opinion this mechanism is similar to the formation of polynuclear hydroxocomplexes in aqueous solution.
This study deals with the effect of hydrolysis process with formation of mononuclear hydroxocomplexes on their sorption on ion exchange resin and silica gel. The mononuclear hydroxocomplexes are a small part of the variety of possible complexing forms that may be present in aqueous solution. Nevertheless, even on this example it is seen that the knowledges on the distribution of radionuclides by their forms in solution and the sorption behaviour of each complex with respect to sorbent are necessary for creation of effective technologies for treatment of liquid radioactive wastes since not always the sorption properties of radionuclide complexing forms may be predicted from the theory.
We believe that the following scheme may prove to be useful for the development of such technologies:
The effect of the following factors on the state and sorption behaviour of the metals in aqueous solution is to be investigated:
REFERENCES
1. Yu.P. Davydov, The state of radionuclides in aqueous solutions, Minsk,Nauka i Technika, 1978.