USE THE REGULARITY OF CHEMICAL BEHAVIOUR OF
FERROUS SALTS IN AQUEOUS SOLUTIONS FOR LIQUID
WASTE TREATMENT PROCESSES
Toropov I.G., Toropova V.V., Davydov Yu.P., Davydov D.Yu., Zemskova L.M.
Institute Radioecological Problems NAS B
Minsk, Belarus
Grebenkov A.J.
Institute of Power Engineering Problems NAS B
Minsk, Belarus
Ridley M.N.
Lawrence Livermore National Laboratory
Livermore, CA, USA
Efimov K.M.
Institute of Ecology
Technological Problems at International Academy of Information Processes and Technologies
Moscow, Russia
ABSTRACT
The effects of some complexing anions and reducer (thiourea) on the formation of iron polynuclear forms in aqueous solution was studied in this work.
It has been shown that polynuclear hydroxocomplexes of iron(III) are effectively detained by semipermiable membranes.
According to the results obtained the polynuclear hydroxoforms of Fe(III) interact with some anions (i.e., JO3-, PO43-, etc.). As a result the anion looses its ability to permeate through the semipermiable membrane in the presence of iron polynuclear forms. this fact can be used for cleaning up aqueous solutions from the complexing anions by membrane methods.
It has been proposed to make use of difference in the behavior of Fe3+ and Fe(CN)63- ions in some reactions of reduction for synthesis of such sorbent as ferrocyanide of iron in solution containing polynuclear complexes of iron(III), what allow one to clean up aqueous solutions simultaneously from radionuclides and some complexing anions, applying this approach.
The obtained data show the possibility of effective application of properties of iron polynuclear complexes for cleaning up aqueous solutions.
INTRODUCTION
Iron is an element widespread in the Earth and widely used in different industrial processes. One of the important applications of iron is that its salts, such as phosphate, cyanide and others, can be used for purification of solution from harmful, including radioactive, substances. Such sorbents are normally used in the solid form.
It is well known fact that iron may form strong complexes with hydroxyl ion in aqueous solution though the practical application of this property is often confined to synthesis of iron hydroxide solid phase. Nowadays the sorption properties of iron hydroxide is widely used for cleaning up aqueous solutions from metal ions and some anions. However, in aqueous solution a range of species may form with increase of pH before sedimentation of iron hydroxide occures:
Fe3+ ® Fe(OH)n3-n ® Fep(OH)q ® colloidal particles ® sediments of Fe(OH)3.
Each member of this sequence possesses its own physico-chemical properties. So, for example, polynuclear hydroxoforms of iron(III) can be detained by semipermiable membrane like particles of colloidal size.
It should be noted that, though hydrolytic properties of Fe(III) are well studied at present, some particularities of its hydrolytic behavior, especially those connected with formation of polynuclear forms in solution, are applied, in our opinion, extremely rare in practice.
The presence of complexing anion may has a substantial effect on the form of iron in aqueous solution. So, the complexing anions may displace OH--ions in coordination sphere of Fe3-cation or be included in the composition of polynuclear complex together with hydroxyl ion.
Some anions form very stable bonds with iron ions. For example, iron forms stable complexes with CN- - anions (Fe(CN)63- and Fe(CN)64-). These complexes can be used for synthesis of sorbents on the basis of ferrocyanides of heavy metals.
So, in our opinion, the knowledges on the conditions of formation and some features of physico-chemical behaviour of each form of iron in aqueous solution may prompt us to creation of improved technologies for treatment of aqueous waste applying the chemistry of ferrous and ferric salts.
EXPERIMENTAL
The effect of complexing anions on the state of Fe(III) in aqueous solution was investigated applying a series of physico-chemical methods - dialysis, ultrafiltration, centrifugation and pH-metric titration. The methods of dialysis and centrifugation were used for determination of the ratio between non ionic (polynuclear, colloidal) and ionic (mononuclear) forms of Fe(III) in solution. The contents of Fe and I were determined using their radioactive isotopes Fe59, I131.
The results of ultrafiltration experiments were presented as the percentage of iron and anion detention calculated by the following equation:
a
u = {(A0 - Ap)/A0} · 100%, whereA0, Ap - concentrations of the investigated substance before and after ultrafiltration accordingly.
The results of dialysis were presented as dialysis coefficients calculated by the following equation:
|
[Fe]inner |
||||||
|
Kd = |
------------- |
, where |
||||
|
[Fe]outer |
[Fe]inner - concentration of Fe(III) mononuclear forms in the "inner" solution and [Fe]outer - concentration of Fe(III) mononuclear forms in the "outer" solution [1].
Percentage of iron in the polynuclear state was calculated, using the dialysis coefficients, by the following equation:
|
1 - Kd |
||||||
|
a p = |
------------- |
[1]. |
||||
|
1 + Kd |
The centrifugation of solutions was carried out using a laboratory centrifuge at 8000 rpm during 30 min. The results were presented as centrifugation sedimentation percentage.
RESULTS AND DISCUSSION
The investigation of IO3-, PO43-, Fe(CN)63--anions effect on the process of hydrolytic polymerization of iron(III) was an objective of this work.
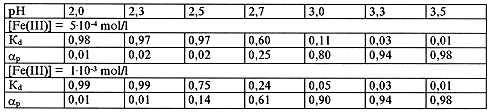
Hydrolysis of iron(III) in a 0.1 mol/l solution of (H - NH4)NO3 was studied, using dialysis method, in a range of iron concentrations 5.10-4 - 1.10-3 mol/l and pH 2.0 - 3.5 in order to determine the conditions of the polynuclear hydroxocomplexes formation (Tabl. I).
From Table I it is seen that dialysis coefficients remain equal 1 before pH 2,3 in the investigated range of iron(III) concentrations. This means that under the above conditions there are no polynuclear complexes of iron in solution. Starting from pH = 2.3 and iron concentration 1. 10-3 mol/l and, accordingly, for iron concentration 5. 10-4 mol/ l - pH = 2,5 the dialysis coefficients become less than 1. This decrease of the dialysis coefficients indicates that the process of hydrolytic polymerization begins in solution under the above conditions.
Table I. Dependence of Iron Polynuclear Forms Percentage on PH of Solution

From the data obtained it follows that at a fixed concentration of iron(III) the percentage of its polynuclear forms in solution grows with increase of pH and there is a certain threshold pH value for each iron concentration at which the polynuclear complexes formation begins in solution.
These data reveal the distribution of iron(III) between its hydrolysed species, and conditions (iron concentration, pH) under which they may exist in solution, and were used in the present work as a basis for investigation of anions effect on the formation of iron polynuclear complexes.
The Effect of JO3--Anion on the Formation of Polynuclear Hydroxocomplexes of Fe(III) in Solution
The presence of complexing anion may influence the formation of iron polynuclear hydroxocomplexes changing the hydrolytic equilibrium in solution. The complexing anions may displace OH--ions in coordination sphere of Fe3-cation by that hindering the formation of polynuclear hydroxocomplexes in which the role of bridges between cations belongs to OH- ion. Besides, at some conditions, the anions may be included in the composition of polynuclear complex together with hydroxyl ion. Such anions will show the properties of the polynuclear complex that is it may be detained by semipermiable membrane or sedimented by centrifugation.
The effect of iodate ion on the formation of iron polynuclear hydroxocomplexes was studied using dialysis method. The results are shown in Tables II, III.
Table II. State of FE(III) and IO3- Ions in Solution According to Dialysis Data.
The Solution Composition: [FE(III)]=5· 10-4 mol/l, [IO3-]=10-4 mol/l
Dialysis of Fresh Solutions
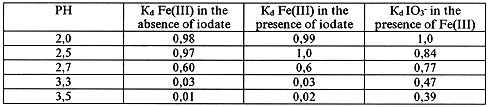
Table III. State of Fe(III) and IO3- Ions in Solution According to Dialysis Data.
The Solution Composition: [Fe(III)=10-3 mol/l, [IO3-] = mol/l
Dialysis of Fresh Solutions
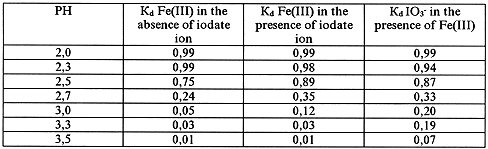
The obtained data show that iodate ion does not influence the formation of iron(III) polynuclear complexes in solution though it may be detained by the semipermiable (cellophane) membrane at the dialysis of solutions containing Fe(III). The detention of iodate ion by the membrane occurs only in the presence of iron polynuclear complexes in solution. This imply that the transfer of iodate ion into nonionic state takes place due to interaction with the iron polynuclear complexes. Probably, the iron polynuclear complexes join the negative iodate ion due to their positive charge.
So, the above shows that it is possible to extract iodate ion from aqueous solutions using the membrane method.
The Effect of PO43-Anion on the Formation of Polynuclear Hydroxocomplexes of Fe(III) in Solution
The dialysis and centrifugation methods were applied for investigation of the phosphate ion and iron polynuclear complexes mutual effect on their state in aqueous solution (Tables IV - VI).
Table IV. Dialysis of Fe(III) in the Presence of Phosphate Ion in Solution.
[Fe(III)] = 10-3 mol/l, pH = 3.0

Table V. Centrifugation of Fe(III) in the Presence of Phosphate Ion in Solution.
[Fe(III)] = 10-3 mol/l, pH = 3.0
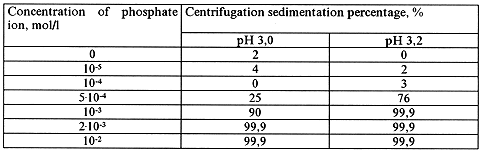
The obtained data show that with increase of phosphate ion concentration in solution sedimentation of Fe(III) occurs at a pH lower than that for iron hydroxide sedimentation. This may imply that both hydroxyl and phosphate ions take part in the formation of sediment. In order to find out whether the phosphate ion participates in the formation of polynuclear forms of iron the influence of already formed iron polynuclear complexes on the state of phosphate ion in solution was studied. The experiment comprised preparation of iron polynuclear complex solution and subsequent addition of the solution containing phosphate ion to it. The pH values of both solutions were equal. The state of phosphate ion was determined by dialysis method. Preliminary study showed that phosphate ion at concentrations 10-7 - 10-4 is not detained by cellophane membrane in solution in the absence of iron(III) ions and its dialysis coefficient is equal 1.
It appeared that in the presence of iron polynuclear forms phosphate ion was detained by cellophane membrane what testifies to joining the iron polynuclear species by phosphate ion (Tab. VI).
Table VI. Dialysis of Phosphate Ions in the Presence of Fe(III) Polynuclear Complexes. pH=3.0
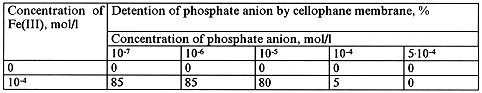
The data show that phosphate ion interacts strongly with the polynuclear forms of iron under the above conditions in aqueous solution. We believe that the observed decrease in phosphate detention at concentrations higher than 10-5 may be explained by saturation of iron polynuclear forms with phosphate-ion.
On the basis of the data presented the centrifugation or ultrafiltration methods may be proposed for removal of iron and phosphate from week acid solutions.
The Effect of Fe(CN)63--Anion on the Formation of Polynuclear Hydroxocomplexes of Fe(III) in Solution
The effect of ferrocyanide anion on the hydrolytic behaviour of Fe(III) cations was studied applying the dialysis method.
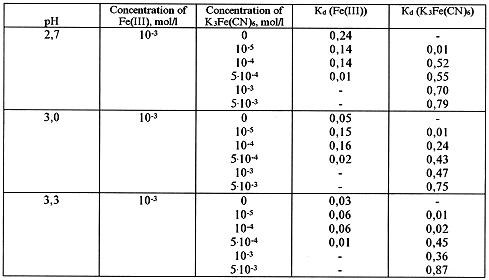
The obtained data, presented in Tables VII - IX, show that the ferricyanide anion has rather a weak effect on the formation of iron polynuclear hydroxoforms.
Table VII. Dialysis of Fe(III) in the Presence of Ferrocyanide Ion in Solution
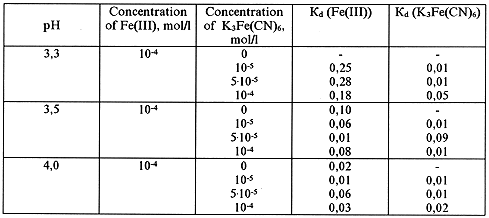
Table VIII. Dialysis of Fe(III) in the Presence of Ferrocyanide Ion in Solution
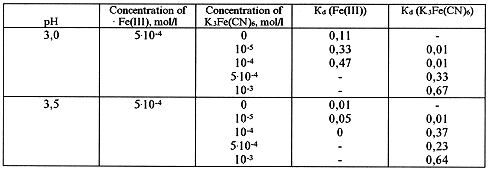
Table IX. Dialysis of Fe(III) in the Presence of Ferrocyanide Ion in Solution
In solutions containing both iron(III)- and cyanide-ions iron may occure either in a form of
Fe3+-cation or Fe(CN)63-anion. If we assume C1 to be the concentration of Fe3+-cation and C2 of Fe(CN)63-anion then the total concentration of iron in solution is C = C1 + C2 and the rate of iron capable of hydroxocomplexes formation can be calculated as C1/(C1 + C2).
The ferricyanide-ion, at its low concentrations, was detained by the semipermiable membrane in the presence of polynuclear hydroxocomplexes of iron(III) in solution. It is difficult to explain this detention on the basis of the data available.
The ultrafiltration data show that the detention of both Fe(III) and ferricyanide ions occurs when concentration of ferricyanide is less or equal to that of Fe(III) ion. No detention was observed in the case when the concentration of ferricyanide ion was more than concentration of Fe3+ ion. This testifies that at high concentrations ferricyanide is in ionic state in solution.
The Effect of Reducer on the Valent State of Fe3+-Cations and of Fe(CN)63--Anions in Solutions
The effect of thiourea on the valent state of free iron(III) in solution was investigated at iron concentrations 5.10-3 mol/l and in a range of pH 1.0 - 3.5. The effect of thiourea on the valent state of iron(III) bound in the complex compound with cyanide-ion was investigated at Fe(CN)63- concentration 6.9.10-3 mol/l and in a range of pH 1.0 - 10.0.
The changing of iron(III) and ferricyanide ion concentrations was observed as a function of time.
The concentration of Fe(III) was measured specrtophotometrically. The capacity of iron(III) ion to form the colored complex with sulphosalicylic acid in aqueous solution was used for its determination [2]. The measurements were taken at the wave length 430 nm.
The determination of ferricyanide ion concentration was carried out also applying spectrophotometry method. The following famous reaction was made use of in order to obtain colored complexes of iron(III). The blue colloidal solution, the molar extinction coefficient of which at the wave length 700-720 nm is about 5000, forms in solution on addition of bivalent iron salt. More detailed the method of ferricyanide ion analysis is described in work [3].
The obtained data on the effect of thiourea on the state of iron(III) and Fe(CN)63- in solution are presented in Tables X - XI.
Table X. The changing of Fe(II) Percentage(%) in the Presence of Thiourea as a Function of Time; Initial Concentration of Fe(III) 5.10-3 mol/l. Concentration of Thiourea 5.10-3 mol/l. Ambient Temperature
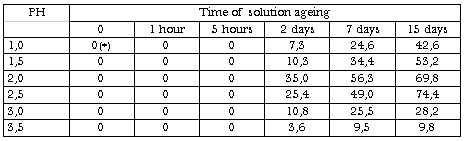
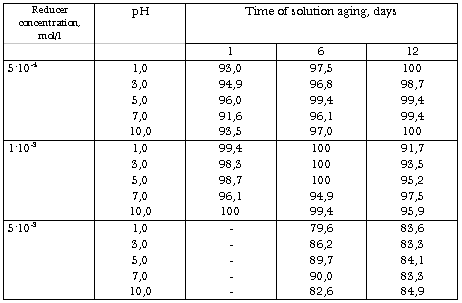
Table XI. The Changing of Fe(CN)63--ion Percentage(%) in the Presence of Thiourea as a Function of Time. Initial Concentration of K3Fe(CN)6 6.9.10-3 mol/l. Ambient Temperature
From tabl. X it is seen that thiourea reduce unbound iron(III) to iron(II). This explains a reduction in detention of iron by semipermiable membrane that was observed under the conditions when in the absence of thiourea the detainable polynuclear complexes of iron form in solution, since iron(II) has a much lesser ability to form polynuclear hydroxocomplexes than iron(III).
However, thiourea had no or little effect on the valent state of iron(III) bound in complex with cyanide-ion in pH range 1.0 - 10 (Tabl.XI).
CONCLUSION
The effects of some complexing anions and reducer (thiourea) on the formation of iron polynuclear forms in aqueous solution was studied in this work.
It has been shown that iron polynuclear forms are extensively detained by semipermiable membranes and that the complexing anion loose its ability to permeate through the membrane in the presence of iron polynuclear forms what testifies to interaction between the complexing anion and polynuclear complex. This fact, in our opinion, can be used for cleaning up aqueous solutions from the complexing anions by membrane or centrifugation method.
It has also been shown that thiourea reduces free Fe(III) to Fe(II) though has no or little effect on the valent state of Fe(III) bound in complex with cyanide-ion. It is possible to make use of this difference in reduction behaviour for synthesis of such sorbent as ferrocyanide of iron in the solution simultaneously containing polynuclear complexes of iron(III):
If the above reaction is performed in an excess of iron(III) then after completion of reaction some iron(III), capable of polynuclear hydroxocomplexes formation, will remain in solution. Since, ferrocyanide of iron is known to be highly effective sorbent with respect to Cs and iron polynuclear hydroxocomplexes may extract some anions, it is possible to clean the aqueous solutions from radionuclides and anions simultaneously applying this approach.
So, in our opinion, the data on hydrolytic behavior of iron(III) in aqueous solution and behavior of different complex forms of iron(III) in different physico-chemical processes, specifically in reactions of reduction, may serve as a basis for development of technologies for: decontamination of liquid radioactive waste, cleaning up the aqueous waste of industrial enterprises and extraction of some anions and cations from aqueous solution.
REFERENCES