SEPARATION OF STRONTIUM FROM LIQUID WASTE BY
PRE-FORMED FERRITE PROCESS
Jae Hak CHEONG
Radwaste Safety Assessment Department
Korea Institute of Nuclear Safety
19 Yusong-gu, Kusong-dong, Taejon 305-338, KOREA
Kun Jai LEE
Department of Nuclear Engineering
Korea Advanced Institute of Science and Technology
373-1 Yusong-gu, Kusong-dong, Taejon 305-701, KOREA
ABSTRACT
In this study, the feasibility of implementing the pre-formed ferrite treatment method into the separation of strontium from liquid radioactive waste has been proved in both theoretical and experimental ways. First of all, by performing a set of experiments with varying the ferrite composition, it turned out that MnO·Fe2O3 has the highest selectivity toward Sr, which has been known to be scarcely removed by the conventional ferrite process. The validity of the proposed reaction mechanisms and the modeling concept was verified by using the experimental data attained from the Sr2+/MnO·Fe2O3 system. The complexation constants log*KSr2+ and log*KSrOH+ have the values of -0.5 and -13.0, respectively. The theoretical maximum Sr-removing capacity of MnO·Fe2O3 is about 0.28g·g-1. However, it turned out that the optimal operating condition is above pH 9, and the decrease of the removal efficiency caused by the disturbing factors are relatively large compared to the Co2+/FeO·Fe2O3 system. In most of the ferrite treatment systems, the plots of logCf vs. -log[H+] can be well-fitted with a quadratic equation. This relation can be used as a system-specific characteristic curve in order to predict the process efficiency of the large-scale ferrite process. It is anticipated that the pre-formed ferrite process can be effectively and directly adopted into pre-treatment stage of the conventional LRWPS or various alkaline (> pH 9) waste streams such as miscellaneous wastewater and floor drains.
INTRODUCTION
It has been reported that the ferrite process has lots of advantages compared to conventional ion exchange or chemical precipitation in many aspects: that is, various heavy metal cations can be simultaneously removed in one step, small amount of solids are to be remained, and so forth [1]. Accordingly the ferrite process has been widely adopted into various metal-contaminated wastewater treatment plants.
Most of the previous studies, however, have been only concentrated on the experimental works for transition metal removal using iron ferrite as sorbent. Though it is known that the reaction mechanisms in the ferrite process are quite complex, the exact mechanisms over the whole process have not been well understood. Therefore mathematical modeling studies on the ferrite process have been quite limited. In order to implement the ferrite process in the liquid radioactive waste processing system (LRWPS), the following limitations should be preferentially settled: (1) deficiency in knowledge about the reaction mechanism, (2) absence of adequate model, and (3) difficulty in removing non-transition metal elements [2].
We have been performing a series of studies for proving the feasibility of adopting the ferrite method into the LRWPS in both theoretical and experimental ways. In our previous works for Co2+/FeO·Fe2O3 system, it was found out that surface complexations, ion exchange, and bulk precipitation play important roles in the whole ferrite process [2,3].
The main objectives of this study are to find a strontium-selective ferrite material and to propose a ferrite process which can separate strontium (a representative non-transition metal element and major radionuclide) from liquid radioactive waste with high efficiency. In addition, the reaction mechanisms will be elucidated and the validity of the proposed model for Sr-removing ferrite treatment system will be also shown. If the non-transition metal elements (e.g. Sr, Cs, etc.) can be effectively removed by the ferrite process, it is expected that the liquid radioactive waste consisting of various metal cations can be treated in one step of the ferrite process.
SELECTION OF SORBENT AND MODELING CONCEPT
Manganese Ferrite
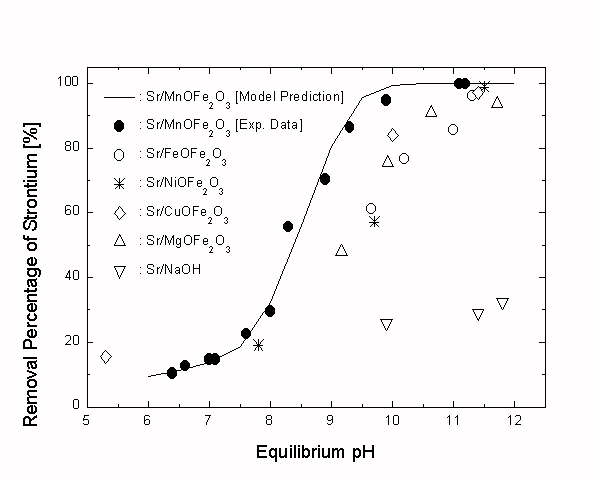
The crystal structure of a ferrite can be presented as MSTIIO·Fe2O3 where MSTII represents various divalent structural metal cations such as FeII, CoII, NiII, MnII, CuII, MgII, and so forth [1]. In most of the previous studies on the ferrite process, the iron ferrite (FeO·Fe2O3) has been exclusively used as sorbent and it was concluded that only the transition metal elements can be effectively removed by the ferrite process. However it is expected that the sorption characteristics along with physical properties of the ferrite material can be varied by changing the structural divalent metal element. In this study, the performances of various ferrite materials (FeO·Fe2O3, MnO·Fe2O3, NiO·Fe2O3, CuO·Fe2O3, and MgO·Fe2O3) as a strontium-removing sorbent were experimentally tested and it was found that the manganese ferrite (MnO·Fe2O3) is the most Sr-selective material among them.
These ferrites were prepared by the wet-neutralization method with 1.00´10-4 mol of Fe3+ (as FeCl3·6H2O) and 5.00´10-5 mol of MST2+ (as FeSO4·7H2O, CuCl2·2H2O, MgCl2·6H2O, and Ni(NO3)2·7H2O, respectively) for each batch system. All the experimental conditions were the same as Sr2+/MnO·Fe2O3 system. The experimental results are illustrated in Fig. 1 showing that the ferrite treatments are more efficient than the simple chemical precipitation method (i.e. Sr2+/NaOH system). In addition, it turned out that the MnO·Fe2O3 is the most Sr-selective sorbent among the ferrite materials tested in this study. Accordingly, it is proved that the physicochemical properties along with metal-removing characteristics of the ferrite materials can be altered by replacing the MST2+. This also implies that the removal efficiency toward a specific waste metal cation can be enhanced by adequate selection of the ferrite composition.

Fig. 1. Selectives of Sr2+ onto various ferrite materials and effect of equilibrium pH on the Sr2+ removal in the Sr2+/MnO.Fe2O3 system (the solution pH ranges 6.4 to 11.2).
Modeling and Reaction Mechanisms
The aquatic chemistry of Sr2+ is much simpler than that of Co2+. In opposition to Co2+ which easily forms cobalt ferrite (i.e. CoO·Fe2O3), Sr2+ cannot form the ferrite like SrO·Fe2O3, due to the size-exclusion principle. Unlike most of transition metal cations such as Co2+, the aquatic chemistry of Sr2+ is very simple. It has been known that Sr2+ forms only a mononuclear hydrolysis product, Sr(OH)+, depending upon the equilibrium pH. The hydrolysis reaction of Sr2+ can be written as:
|
|
(1) |
|
|
(2) |
In our previous study, it was proved that the formation of Co(OH)2(s) is an important reaction mechanism in the Co2+/FeO·Fe2O3 system. However, Sr2+ does not form any neutralized species such as Sr(OH)2, even at very high pH condition. Therefore, precipitation of Sr2+ as Sr(OH)2 must be excluded in the modeling studies. The mono-hydrolyzed ion, Sr(OH)+, is a kind of aqueous species and cannot directly contribute to the strontium removal from aqueous waste solution. Accordingly, it is anticipated that the reaction mechanisms in the Sr/MnO·Fe2O3 system are relatively simple compared to those in the Co/FeO·Fe2O3 system. However, Sr(OH)+ would play an important role in the pre-formed ferrite treatment through the adsorption onto the surface sites of MnO·Fe2O3.
Ultimately, only three reactions were proposed as important mechanisms in the Sr/MnO·Fe2O3 system as follows:
(a) surface complexation of divalent strontium cation, Sr2+
|
|
(3) |
|
|
(4) |
(b) surface complexation of mono-hydrolyzed species of strontium, Sr(OH)+
|
|
(5) |
|
|
(6) |
(c) participation of bidentate surface site
|
|
(7) |
|
|
(8) |
where SOH and SO- represent neutral and negative surface sites of the sorbent, respectively, and the subscript, s, denotes the surface species.
In this study, we have adopted the basic principles of the surface complexation model (SCM) and the triple layer model (TLM) in order to simulate Sr2+/MnO·Fe2O3 system [4]. Accordingly, amphoteric properties of the surface sites and electrostatic effects on the surface species were considered. More detailed descriptions have been already given in a variety of literatures. The electrical charge densities at o- and b-planes can be given as follows:
|
|
(9) |
|
|
(10) |
The material balances for the surface sites and Sr species can be written by:
|
|
(11) |
|
|
(12) |
The concentrations of Sr-containing surface sites can be rewritten by considering electrostatic terms, mass-action laws, and material balances as follows:
|
|
(13) |
|
|
(14) |
|
|
(15) |
Finally, the distribution coefficient, kd (dm3×kg-1), can be written as:
|
|
(16) |
where V is the total volume of waste solution in dm3, and m is the dry mass of MnO·Fe2O3 used in kg. The removal efficiency can be also simply represented by removal percentage, P (%), as:
|
|
(17) |
Calculation Method
All the equations and constraints describing the whole reaction system of the pre-formed ferrite process (i.e. MW2+/MSTO·Fe2O3 system) have been given in the previous section. And the problem can be reduced to a relatively simple chemical equilibrium problem including the interfacial phenomena. That is, the equations can be numerically by using a Newton-Raphson method at a certain condition (e.g. pH, concentrations of each chemical species, etc.), when a series of required parameters are to be known. The basic concept and methodology for calculating the chemical equilibrium problem has been expounded in a variety of literatures [2,4].
The chemical equilibrium problems have been of concern for the last few decades, in the fields of geochemistry, environmental chemistry, civil engineering, and so forth. Accordingly, a variety of studies for developing the computation tools and database have been performed, and lots of very sophisticated computer codes have been developed. Among them, the MINTEQA2 developed and officially certified by the U.S. EPA was used as a basic computation tool [5]. In this study, the computational part of the MINTEQA2 Version 3.11, without modification, was adopted into the model calculation procedure. However, the thermochemical database was partly revised in order to consider some additional reactions which are not included in the original version of the MINTEQA2.
EXPERIMENTAL PROCEDURES
The sorbent, MnO·Fe2O3, was prepared by wet-neutralization method at room temperature, in which the spinel ferrite is to be pecipitated in an aqueous solution containing manganese and ferric ions by adding an alkaline reagent:
|
|
(18) |
Both 5.00´10-5 mol of Mn2+ (as MnCl2·4H2O) and 1.00´10-4 mol of Fe3+ (as FeCl3·6H2O) were dissolved in 0.01 dm3 of water, and then NaOH was added into the solution until the pH was set to the target point (pH 11.0±0.1).
A series of batch experiments was carried out in 0.1 dm3 polyethylene bottles at 25.0±0.1 oC. The pre-formed MnO·Fe2O3 was added into 0.005 dm3 of 2.00´10-3 M (» 175 ppm) Sr solution without any additional treatments. Then the total volume of the mixed solution was finally raised to 0.05 dm3. The pH of the aqueous solution was adjusted to reach the equilibrium pH of 10.5±0.1 (otherwise mentioned). Therefore, the initial concentration of Sr solution was set to 2.00´10-4 M (» 17.5 ppm). The solution was equilibrated by shaking for 4 h at 90.0 rpm, and then complete separation of the solid and aqueous phases was accomplished by high-speed centrifugation for 10 min at 12,000 rpm. The concentration of Sr species remained in the supernant was analyzed by atomic absorption spectrophotometer (AAS; Perkin Elmer 3100) N2O-acetylene flame analysis technique at 460.7 nm.
RESULTS AND DISCUSSION
It was shown that Sr existing in simulated liquid waste can be effectively removed using the pre-formed ferrite process with relatively small amount of MnO·Fe2O3. In addition, various factors and operating conditions affecting the Sr-removal efficiency were investigated.
Reaction Kinetics and Thermodynamics
More than 90% of the initial amount of Sr was removed only after 30 min and the reaction system reaches a quasi-equilibrium state after 240 min. Even though the overall reaction proceeds more rapidly than conventional adsorption, the reaction is relatively slow compared to the Co2+/FeO·Fe2O3 system where over 99% of initial Co was removed only after 3 min.
The overall reactions turned out to be endothermic (DHo = 13.9 kJ·mol-1) and the value also implies that the overall reaction belongs to chemisorption or ion exchange which is compatible with the reaction mechanisms proposed in this study. It also turns out that the standard enthalpy change of the Sr2+/MnO·Fe2O3 system (13.9 kJ·mol-1) is much smaller than that of Co2+/FeO·Fe2O3 system (25.0 kJ·mol-1).
Experimental PH-Edge and Model Application
As shown in Fig. 2, The removal efficiency increases with pH and the slope increases sharply in the medium pH region (i.e. pH 7.5 to 9.5). The low removal efficiency in the low pH region can be explained by the competitive adsorption of H+ and Sr2+ onto the same adsorption site. The values of parameters and experimental conditions of this study are displayed in Table I.
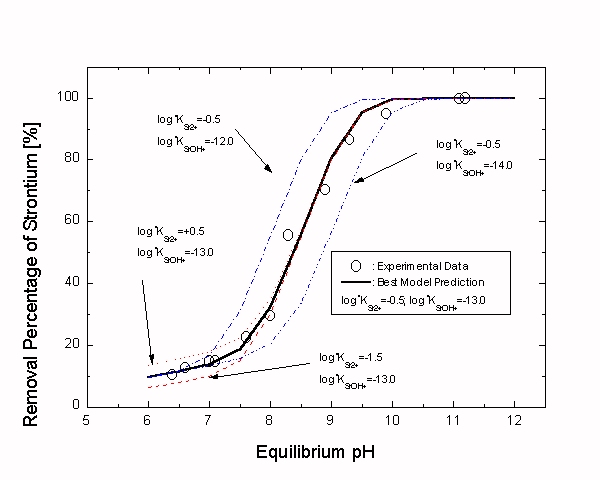
Fig. 2. Result of the sensitivity analysis about the pH-edge prediction.
Table I. General Experimental Conditions and Values of Parameters in This Study
|
Parameter |
Value |
Parameter |
Value |
|
T |
25 ° |
[Sr2+]TOT |
2.00 ´10-4 M |
|
V |
5.00 ´10-2 dm3 |
[SOH]TOT |
1.00 ´10-3 M |
|
As |
600 m2·g-1 |
Cs |
0.231 g·l-1 |
|
C1 |
1.40 F·m-2 |
log *KSr2+ |
-0.5 |
|
C2 |
0.20 F·m-2 |
log *KSrOH+ |
-13.0 |
The Sr2+/MnO·Fe2O3 system was also successfully simulated by the ESCM and the values of surface complexation constants log*KSr2+ and log*KSrOH+ were determined as -0.5 and -13.0, respectively. In the process of pH-edge prediction, it was found out that contribution of the bidentate surface sites would be negligible. It can be concluded, as the result of sensitivity analysis on the free parameters, that log*KSrOH+ is a predominant factor. Even though the value of log*KSr2+ is much larger that that of log*KSrOH+, the effect of log*KSrOH+ is much stronger than that of log*KSr2+. That is, the overall shape of the pH-edge is to be mainly determined by the value of log*KSrOH+.
The removal efficiency of Sr observed in this study is much higher than those of conventional adsorption reactions. For instance, Todorovic et al. have shown that 22.8% of initial Sr is removed at pH 8.9 in Sr2+/natural magnetite (i.e. Fe3O4) system and only 5% is removed even at pH 9.4 in Sr2+/natural hematite (i.e. a-Fe2O3) system [6]. The relatively high Sr removal efficiency attained in this study (about 70% at pH 8.9) can be ascribed to the fact that the freshly prepared MnO·Fe2O3 has higher specific surface area and more active surface sites than the naturally occurred or aged Fe3O4.
Kinniburg et al. (1975), Kolarik (1961), and Dzombak and Morel (1989) have studied on the adsorption phenomena of Sr2+ onto hydrous ferric oxide [4,7,8]. As listed in Table II, the surface complexations assumed by the investigators are somewhat different from those of this study. Comparing with other investigators' experimental data sets, however, it can be at least concluded that the values of the surface complexation constants calculated in this study are quite reasonable.
TABLE II. Comparison of the Surface Complexation Constants for the Strontium Removal Systems
|
Sr2+/MnO·Fe2O3 System (This Study) |
||||||||||||
|
log*KSr2+ |
-0.5 |
log*KSrOH+ |
-13.0 |
|||||||||
|
Sr2+/Hydrous Ferric Oxide System |
||||||||||||
|
Kinniburgh et al. (1975) |
Ref. [7] |
log*KSr2+ |
+4.94 |
|||||||||
|
Kolarik (1961) |
Ref. [8] |
log*KSr2+(S) |
+4.95 ~ +5.30 |
|||||||||
|
log*KSr2+(W) |
-6.80 ~ -6.20 |
|||||||||||
|
log*KSrOH+ |
-17.75 ~ -16.82 |
|||||||||||
|
Dzombak & Morel (1989) |
Ref. [4] |
log*KSr2+(S) |
+5.01 |
|||||||||
|
log*KSr2+(W) |
-6.58 |
|||||||||||
|
log*KSrOH+ |
-17.60 |
|||||||||||
Characteristic Curve
It was also found out that the plot of logCf vs. pH (in pH 6.5 to 11) follows a convex-type quadratic relation as:
|
|
(19) |
It was found out that the plot of logCf vs. pH can be a characteristic curve for each ferrite treatment system. Furthermore, the relation can be used for predicting the overall performance of each ferrite process. Quantitative study on this subject is worthy of further investigation. So to speak, it is needed that a certain correlation between Cf and various operational conditions such as Co is to be elucidated.
Other Factors
The theoretical maximum Sr removal capacity, Xmax, calculated from the inverse of the slope in Langmuir isotherm, is 6.5 eq.·g-1 (or 286 mg·g-1). The disturbing effects caused by solution ionic strength, chelating agent (EDTA), and competing cation (Ca2+) were relatively small compared with the conventional adsorption of Sr2+ onto the surface of inorganic adsorbents (see Fig. 3). The effects observed in the Sr2+/MnO·Fe2O3 system, however, are larger than those observed in the Co2+/FeO·Fe2O3 system.
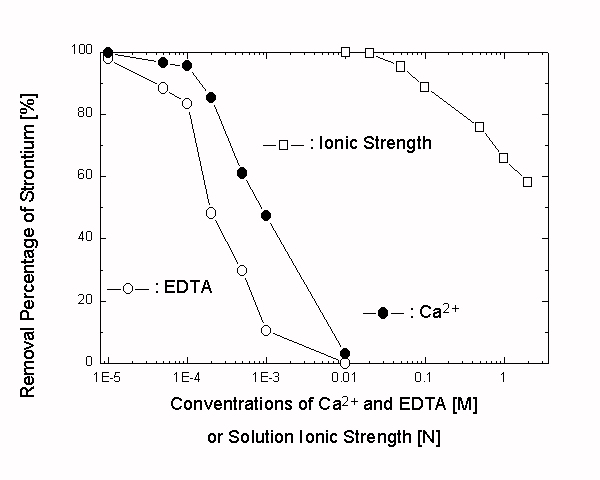
Fig. 3. Effect of the solution ionic strength on the Sr2+ removal in the Sr2+/MnO.Fe2O3 system (the ionic strength is controlled using NaCl and ranges 0.01 to 2.00 N).
CONCLUSION
As the result of the selectivity analysis of various ferrite materials to the strontium species, the manganese ferrite (i.e. MnO·Fe2O3) was selected as the most Sr-selective sorbent. It has been also proved that the reaction mechanisms involved in the Sr-removing system is much simpler that those involved in the Co-removing ferrite treatment system. That is, only the surface complexations of Sr2+ and SrOH+ are two of the most dominant reaction mechanisms in the system.
The optimal operating conditions is above pH 9 and the surface complexation constants are log*KSr2+ = -0.5 and log*KSrOH+ = -13.0, respectively. The maximum Sr-removing capacity of the MnO·Fe2O3 is about 30% of its sorbent mass. The affinity of the MnO·Fe2O3 toward Sr2+ is almost same as that of EDTA to Sr2+. The system-specific characteristic curve of logCf vs. pH is to be a concave-type quadratic relation.
Due to the innate characteristics of the ferrite process (i.e. ferrite precipitation condition), alkaline waste streams are to be preferred. In these days, it has been reported that the high pH operation of the nuclear reactor by water chemistry control would contribute to reduce the ORE. If the high pH reactor operation is implemented, the pH of the LRW is also increase and the preference of the ferrite process is to be improved. In addition, a series of studies on the vitrification of the LLW has been recently performed. It has been also reported that the secondary solid waste remained after ferrite treatment is easily and safely vitrified without complex conditioning. Therefore, the applicability of the ferrite process is to increase, if the process is connected with the vitrification system.
As previously mentioned, the target waste stream where the ferrite can be adopted, is to be slightly alkaline, and it is anticipated that the process efficiency can be improved with increasing the pH. Among a variety of waste streams, it has been reported that floor drain and miscellaneous wastewater are highly alkaline (i.e. pH > 11 or 12). It can be anticipated that the ferrite can be formed in the above-mentioned waste streams without any additives, and the economical efficiency of the process would increase. In addition, the ferrite process can be implemented into the pre-treatment system of the conventional ion exchange systems as stated in the EPRI report, NP-5786 [9].
The feasibility of the application of the pre-formed ferrite process has been proved in this study. However, there still exit a few items to be investigated in order to implement the full-scale pre-formed ferrite process into the LRWPS. First of all, a continuous operation system such as column-type reactor is to be developed. Also, Cs- and I-selective ferrite materials should be experimentally determined and their related system parameters are to be elucidated. Finally, a series of economic analysis is required to compare the relative economical efficiency of the process with other alternatives ranging from the conventional ion exchange bed to the chemical precipitation.
REFERENCES